
Las Vegas - A Web site created to support a public-relations campaign about heartworm was unveiled at the recently concluded Western Veterinary Conference.

Las Vegas - A Web site created to support a public-relations campaign about heartworm was unveiled at the recently concluded Western Veterinary Conference.
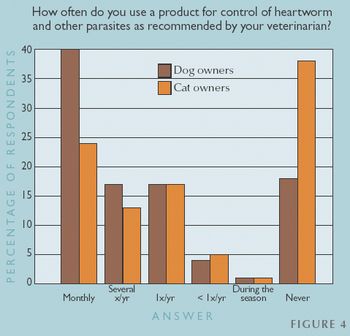
Not surprisingly, clients who visit their veterinarians at least yearly are more likely to use parasite control products, be concerned about the ramifications of parasites, and practice good hygiene to prevent parasite transmission from animals to people.
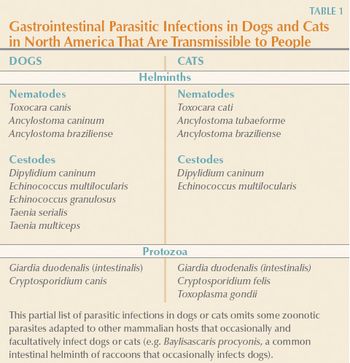
Veterinarians in practice are on the front lines in preventing transmission of pet-associated zoonotic parasite infections because of their knowledge of the potential risks and through their contact with pet owners.
Careful attention is needed both to prevent tapeworm infections and to ensure prompt, effective treatment when infections do occur.

The risk of a dog's being infected with heartworm disease each year is 250,000 out of 50,000,000; this translates to one in 200 dogs becoming infected each year.

Heartworm disease manifests quite differently in cats than in dogs and has an altered infective cycle.

Client education is the single-most important tool for maintaining a relationship that draws clients back to your veterinary practice for information on parasite prevention and treatment products.

Efforts must now focus on increasing awareness of the zoonotic risks associated with many of these parasites and improving pet owner compliance.

To increase parasite prevention and control compliance, make sure the doctors are in agreement about the standards of care and team members know what to say to clients.

Testing, treatment, and public health all play a part.
Orlando - With only 4 percent of cats estimated to be on heartworm medicine, a new campaign from the American Heartworm Society (AHS) aims to change what officials are calling an abysmal prevention rate.

SHAWNEE MISSION, KAN. - 1/31/2007 - Bayer Healthcare LLC recently received Food and Drug Administration (FDA) approval for a new topical product targeting fleas, heartworms and intestinal nemotodes for both dogs and cats. Bayer officials report the approval and subsequent launch signal an important development for broad-spectrum parasite control. Advantage Multi-TM for Dogs Topical Solution and Advantage Multi-TM for Cats Topical Solution are available by prescription only through licensed veterinarians.

This article reviews the recent literature, common clinical presentations, and current recommendations on diagnosing and treating knemidocoptiasis.
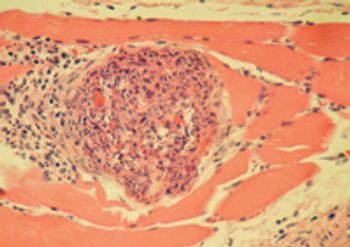
A 2.5-year-old spayed female mixed-breed dog was presented to Claiborne Hill Veterinary Hospital for evaluation of a one-day history of pain when walking.

BEL AIR, MD - 11/28/06 - A September national survey of more than 1,000 dog and cat owners conducted for the nonprofit Companion Animal Parasite Council (CAPC, www.petsandparasites.org) revealed that while many people are aware that children are especially at risk of being affected by zoonotic disease caused by the transmission of parasites to humans by pets, they appear indifferent to the risk.

Most clients would be pretty grossed out to find a flea or tick on their pets. But they don't always take all the steps to protect their pets from infestations. That's where you come in. You want to start pet owners off on the right paw, so begin discussing parasite control the first day clients visit with their new pets.

In the article "Optimize intestinal parasite detection with centrifugal fecal flotation," which appeared in the July 2006 issue, the veterinary distributor for centrifuges manufactured by LW Scientific was not listed.

Gaithersburg, Md.- When fibrosis hardened Pat Dickinson's lungs in late 2004, doctors struggled to isolate her illness. Two years later, the 55-year-old dog owner credits a veterinarian's education for avoiding a lung transplant and even death.
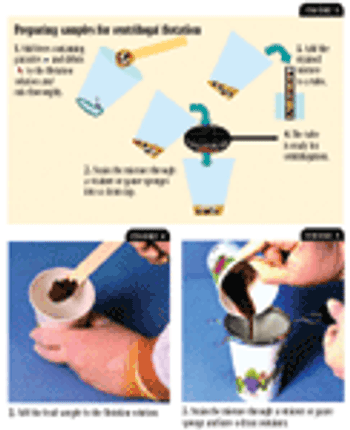
Failure to use best-practice techniques, such as centrifugation, when conducting fecal flotation procedures can result in failure to detect parasite stages in fecal samples. In this article, we review the basics of fecal flotation techniques and describe step-by-step procedures for conducting accurate and effective centrifugal flotation procedures.

Fecal egg counts (FEC) in horses are incredibly consistent for an individual horse, as shown recently from a three-year study in Denmark.

Is your message about the risks of parasites reaching clients?

Not all horses are affected the same, probably 20 percent of horses shed 80 percent of the eggs.

A few years ago, our veterinarians and licensed veterinary technicians learned that centrifugation of fecal samples was the superior test for diagnosing gastrointestinal parasitism.
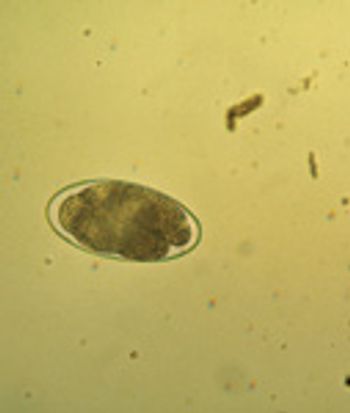
Overcoming clients' preconceived notions can be difficult, especially as newer research suggests that treating horses as individuals might be more effective than a shotgun deworming regimen.

With the increasing numbers of meat goats in the United States, many bovine practitioners face questions about goat healthcare targeted toward internal parasite treatment and control.