
IDEXX Laboratories Inc. received approval from the U.S. Food and Drug Administration (FDA) to market and sell Surpass? (1% diclofenac sodium) Topical Anti-Inflammatory Cream, a new treatment for horses with pain and lameness due to osteo-arthritis.

IDEXX Laboratories Inc. received approval from the U.S. Food and Drug Administration (FDA) to market and sell Surpass? (1% diclofenac sodium) Topical Anti-Inflammatory Cream, a new treatment for horses with pain and lameness due to osteo-arthritis.
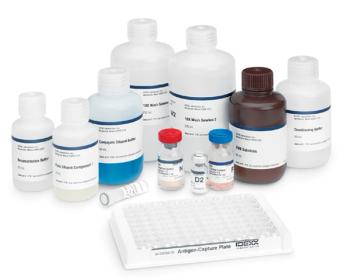
IDEXX Laboratories Inc. received approval from the U.S. Department of Agriculture (USDA) to produce and sell the IDEXX HerdChek? BSE Antigen Test Kit to USDA-approved laboratories.
The role of insects in the virus' spread among farms is being examined.

NEW YORK - Pfizer Animal Health received approval from the Food and Drug Administration (FDA) for Simplecef? (cefpodoxime proxetil) tablets, the only oral cephalosporin approved to treat canine skin infections (wounds and abscesses) with the ease of once-daily dosing.

The NCBA applauds the caution given to test-result disclosure.

LEXINGTON, KY.-The U.S. District Court for the Eastern District of Kentucky issued a seizure warrant Aug. 11 at the request of the Food and Drug Administration (FDA) for various compounded drug products at BET Pharm LLC, in Lexington, Ky.

ITASCA, ILL.-Equine Data Systems International, Inc. (EDSI) purchased the Equine Data Management Software (EDMS) and all of its related materials.

WASHINGTON-The U.S. Food and Drug Administration's (FDA) recent move against compounding drugs from bulk for use in nonfood animals has lured pharmacy groups and organized veterinary medicine to Washington in opposition.

WASHINGTON -The U.S. Supreme Court will decide whether or not the beef checkoff program violates the U.S. Constitution.

WESTBROOK, MAINE-IDEXX Laboratories, Inc., received approval from the U.S. Food and Drug Administration (FDA) to market and sell Surpass? (1% diclofenac sodium) Topical Anti-Inflammatory Cream, a new treatment for horses with pain and lameness due to osteoarthritis.

Washington-As the U.S. Food and Drug Administration's (FDA) labors to restrict compounded drugs for nonfood animals, its efforts are preceded by a 2002 Supreme Court case in which justices criticized the agency for restricting pharmacists' free speech rights.

Washington-Agriculture Secretary Ann M. Veneman announced an awareness campaign entitled "Biosecurity for the Birds," to educate noncommercial bird owners on avian health and poultry diseases.

USDA orders inquiry into rendering incident

Washington-The Food and Drug Administration (FDA) has issued a handful of warning letters to compounding pharmacies working with human and/or animal drugs and at least two written warnings to veterinary practices.

Las Vegas-The U.S. Drug Enforce-ment Administration (DEA) doesn't do spot checks or make social calls. If agents or investigators visit a veterinary practice, there's a problem.

Tacoma, Wash.-In an unprecedented ruling, the Washington Court of Appeals has determined how much pain is needed for an animal cruelty conviction.

Washington-The Environmental Protection Agency (EPA) issued a stop-sale, use and removal orders to retailers and other distributors of counterfeit pesticide products for fleas and ticks.

The FDA Investigations Operations Manual includes revised instructions regarding the release of information.

Raleigh, N.C.-The U.S. Department of Agriculture (USDA) has awarded the State Animal Response Team (SART) a $250,000 grant to reproduce its model of animal emergency response in other states.

Washington, D.C. -A peer-reviewed article raising concern that the banning of antibiotics in food animals may harm both human and animal health, is drawing criticism from the Food and Drug Administration (FDA).

Rockville, Md.-The Food and Drug Administration (FDA) unveiled new controls to help prevent BSE from ever taking a foothold in the United States.

Washington-In the wake of the country's first case of mad cow disease, the United States Department of Agriculture's (USDA) ruling to ban all non-ambulatory, disabled livestock from being slaughtered may mean that the government will rely on veterinarians and producers to get access to test animals at highest risk for mad cow disease.

Scour Bos 9, from Novartis Animal Vaccines, received United States Department of Agriculture approval for administration at eight to 16 weeks prior to calving as an initial dose the first year of vaccination.

Washington-The United States Department of Agriculture (USDA) unveiled its "unified" food safety research agenda.

IDEXX Laboratories, Inc. has been approved by the U.S. Food and Drug Administration (FDA) to market and sell NavigatorӨ (32 percent nitazoxanide) antiprotozoal oral paste treatment for equine protozoal myeloencephalitis (EPM).

Des Moines-Food and Drug Administration (FDA) officials eased an Iowa crackdown on pet foods containing glucosamine and chondroitin last month with its resolution to review animal supplements, stripping the issue from the state's jurisdiction.

User fees to hasten drug approvals for the veterinary market just became a reality.

User fees to hasten drug approvals for the veterinary market just became a reality.

The U.S. Food and Drug Administration granted marketing approval for Metacam Injectable, a non-steroidal anti-inflammatory drug (NSAID), made by Boehringer Ingelheim Vetmedica (BI) and being distributed by Merial. Coupled with Metacam Oral Suspension, the injectable offers an effective source for relief of pain associate with canine osteoarthritis, the company says.

Rockville, Md.-In the wake of a Food and Drug Administration (FDA) draft risk assessment on the safety of animal cloning, the FDA's Veterinary Medical Advisory Committee (VMAC) agreed that products generated from clones would likely be safe to eat but some panel members expressed concern on the risks to animal welfare.