Injectable heartworm preventive is approved for dogs 12 months of age and older and provides 12 months of protection.

Injectable heartworm preventive is approved for dogs 12 months of age and older and provides 12 months of protection.

The bipartisan bill would improve public health preparedness and bolster the effectiveness and efficiency of the U.S. governments response to emergencies.

Agency looks for links between certain ingredients, specific brands and veterinarian-diagnosed cardiac issues in the 500-plus case reports it has received.

A primer on Public Service Loan Forgiveness from the AVMA Econ team.

Anivive Lifesciences starts with unmet therapeutic needs and employs high-tech research methods to identify compounds that could meet those needs.

The Texas Supreme Court has reaffirmed the decision to hold Kristen Lindsey, DVM, accountable for her actions in 2015 that left a cat dead and sparked international outrage.

Catastrophic injuries peaked in late February and March 2019; veterinarians point to multiple factors, call for further investigation.

Governor holds press conference at VCA hospital in Omaha objecting to proposed bill.

Passage of the 2018 Farm Bill and growing consumer interest in cannabis and cannabis-derived products have prompted the FDA to redouble its efforts to protect public health.

Controversial government research has come to a grinding halt, thanks to a watchdog group.

Hedgehogs are so cute that they can make you sick, literally. Here's what the CDC wants you (and your veterinary clients) to know.

Its investigation is ongoing and the agency hasnt changed its recommendation to pet owners whose pets are not ill.

The pet food recall announced by Hills on January 31 has left untold numbers of pet owners devastated and outraged, and many are now seeking justice.

Purdue veterinary researchers document service dogs impact on psychosocial health of recipients, family members.

Florida lawmakers hope to close loophole making videosbut not torturea national felony.

American Horse Council, AAEP, USDA pledge to keep U.S. horse herd healthy in case of a disease outbreak.

Cannabis sativa L no longer defined as illegal substance as long as products derived from it contain less than 0.3% THC.

Human medicine provides a cautionary tale.

Coverage options, halted in 2013 after regulatory changes, will be available to some members and their employees starting in July.
Do veterinary practices have a role in alleviating the national crisis? The feds say yes.

Parasiticide research aims to validate alternatives to use of animal subjects in some bioequivalence trials.
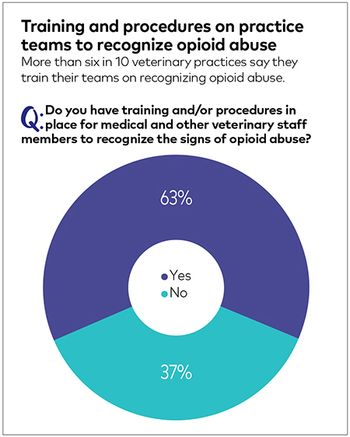
Industry study reports clinicians frustration with curtailed pain management options for surgery and treatment of severe injuries.

High levels of microbial contamination have rendered the product unsafe.
Equine, swine products used by veterinarians and livestock handlers to regulate estrus may cause reproductive disorders in people.

Products cannot be guaranteed sterile; company has failed to issue voluntary recall.

Missouri official reaches out after receiving calls from practitioners questioning articles premise.

Low levels of pentobarbital discovered in Gravy Train, Kibbles 'N Bits and other brands; company traces problem to beef fat.

Deal expected to close by the end of General Mills fiscal year.

Immunotherapy product may delay or prevent metastatic disease and prolong overall survival in veterinary patients.

States have begun legalizing medical marijuana for human patientswill pets see the benefits as well?