
Credelio Quattro (Elanco) was previously approved by the FDA for treatment of infestation for 6 other parasites in dogs.

Credelio Quattro (Elanco) was previously approved by the FDA for treatment of infestation for 6 other parasites in dogs.
The approval of Emeprev will help veterinary professionals by providing an in-clinic antiemetic option.

The product is also indicated treatment and control of cattle fever tick.

The FDA’s new draft guidance outlines when monoclonal antibody developers can reduce or eliminate 6-month primate studies

The FDA determined that Credelio Cat may be effective in treating New World screwworm infestations in cats and kittens.

The announcement comes as raw pet food products have tested positive for bird flu amid fall migratory season.
This completion is a crucial requirement in the approval process for a drug.
Solovecin by Dechra is a generic antibiotic for treatment of skin infections in dogs and cats.

Felixvet has announced the approval for a bioequivalent product to Simplicef (Zoetis).

Consumers failed to adequately plead consumer fraud, product consumer fraud, and warranty claims.

A West Virginia veterinarian faces nearly $1 million in penalties for failing to account for controlled substances and allegedly falsifying records.

The Nature’s Own Pet Chews Bully Bites were recalled due to possible Salmonella contamination.

Zoetis' Dectomax-CA1 is the first drug to receive conditional approval in the US for addressing this type of parasitic infestation.

The revision removes language about fatal vaccine-induced disease from the skin allergy drug's label.

The agency found that Gallant’s data provides reasonable expectation of effectiveness.

The parasiticide also treats Dermanyssus gallinae, also known as red mites.

The food was tested prior to a cat testing positive for H5N1 highly pathogenic avian influenza prior to ingesting one of the infected lot numbers.

Gamrozyne is the first generic version of Zactran, a pioneer product.

Two lots of its dog and cat food are being recalled due to Salmonella and Listeria monocytogenes contamination.

The company voluntarily recalled its 2 lots of product due to potential health risks.

The declaration allows emergency use of animal drugs not yet FDA approved for screwworm treatment.

Three different bacteria were found in different dog food products. One is associated with kidney failure and neurologic issues in humans.

This latest approval builds on an earlier liquid formulation of generic methimazole for feline hyperthyroidism, which received FDA clearance earlier this month
The funding will support Gallant’s treatment for refractory feline chronic gingivostomatitis, which is in development.
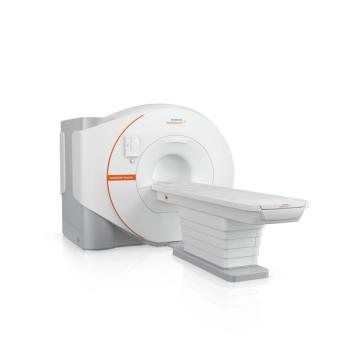
The Magnetom Flow.Ace also uses AI technology for faster scans and image reconstruction.

This approval means that CPMA can be used to prevent canine parvovirus in puppies exposed to the virus.

The FDA was particularly concerned about the selling of unapproved CBD products for food-producing animals.

The latest canine otitis externa medication is the only FDA-approved, 1-dose, in-clinic treatment for Pseudomonas aeruginosa and other pathogens.

Dechra’s Otiserene is a single-dose, long-acting product that utilizes marbofloxacin

This is a groundbreaking step toward advancing public health by using more effective and human-relevant methods instead of animal testing