Meniscal fixation device to aid healing
Columbia, Mo. - A new orthopedic device co-developed and tested at the University of Missouri's (MU) veterinary school received Food and Drug Administration approval for the human market.
COLUMBIA, MO. — A new orthopedic device co-developed and tested at the University of Missouri's (MU) veterinary school received Food and Drug Administration approval for the human market.
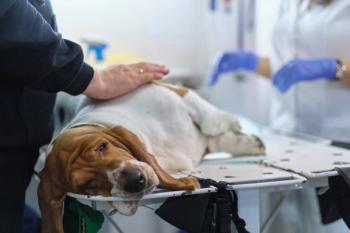
Veterinary surgeon James Cook at MU developed the device with Schwartz Biomedical, LLC.
The device transports blood and cells from the vascular portion of the knee to the avascular portion of the meniscus to spur healing. It works well, according to Cook.
His team performed the surgery on 25 dogs that had worst-case meniscal tears. The dogs studied had complete or partial repair after a few weeks in all cases. Cook's findings were published in the American Journal of Sports Medicine.
Newsletter
From exam room tips to practice management insights, get trusted veterinary news delivered straight to your inbox—subscribe to dvm360.