Dilemmas of degenerative lumbosacral syndrome (Proceedings)
Degenerative lumbosacral syndrome (DLSS) poses diagnostic and treatment dilemmas for the veterinary practitioner.
Degenerative lumbosacral syndrome (DLSS) poses diagnostic and treatment dilemmas for the veterinary practitioner. Aspects of these dilemmas were presented at the 2006 Annual ACVIM Forum (Bagley, Carpenter, Shores – 2006). Diagnosis of DLSS is difficult in distinguishing between significance of clinical signs and associated imaging abnormalities especially while predicting development of DLSS and detecting instability. Treatment protocols need to take into account the dog's activity level and lifestyle. Likewise, the appropriate surgical procedure selected may differ for the pet-owned versus the working dog.
Pathogenesis
Lumbosacral stenosis (cauda equina syndrome, lumbosacral malarticulation and malformation, lumbosacral instability, lumbosacral spondylopathy) is common in middle- to old-aged, large breed dogs and represents a plethora of orthopaedic abnormalities associated with the lumbosacral anatomy (De Risio 2000). Neurologic abnormalities occur coincident with tissue (joint capsule, interarcuate ligament, disc, bone, fibrous adhesions) impingement onto the cauda equina, nerve roots at the level of the foramina, or its vascular supply. The pathologic process begins with Hansen type II disc degeneration followed by osteophyte formation of the L 7-S 1 end plates and articular processes (Chambers 1989). The syndrome is characterized by stenosis of the spinal canal from vertebral subluxation and/or stenotic intervertebral foramen. Attention also has been focused on higher prevalence of DLSS and disk degeneration in German Shepherd Dogs with relation to differences in motion patterns. Shape and orientation of articular facets have been found to be different in GSDs than in other breeds (Seiler 2003). However, the complex studies using of 3-D conformation and motion did not reveal an association between facet joint tropism and disk degeneration and further in vivo studies will be necessary to assess the role of biomechanical factors (Benninger 2006).
Clinical signs
Neuroanatomic localization to the lumbosacral region is determined by the neurologic examination based on signs related to sensory, motor and autonomic dysfunction. Nonspecific observations include reluctance to rise and pelvic limb lameness. Neurologic signs commonly include pain and motor dysfunction as a result of lower motor neuron weakness (sciatic, pudendal, coccygeal nn.). Pain is the most consistent clinical sign and is reflective of compressive or inflammatory processes to pain sensitive structures (nerve root, meninges, periosteum and joints). It is the opinion of the author that the pain primarily originates from nerve root (radicular pain) compression (especially L7). The affected patient often stands with the pelvic limbs tucked under the caudal abdomen to flex the spine and lessen nerve root compression. Pain is manifested by hyperesthesia and/or paresthesia. Hyperesthesia is elicited upon palpation of the lumbosacral joint or by hyperextension of the pelvic limbs causing the spinal region to have lordosis. This accentuates canal stenosis and nerve root compression causing pain. Paresthesia is caused by irritation of the nerve roots without an external stimulus. Clinical signs include biting at the tail, rump and feet. Pain of lumbosacral origin also is exacerbated with exercise as an asymmetric lameness and may reflect neurogenic intermittent claudication. Motor dysfunction varies with severity of neural tissue compression. The patient may have mild to severe gait and postural reaction deficits. The gait is often short-strided. Postural reaction deficits often are asymmetric depending upon the degree of cauda equina compression.
Reflex dysfunction of the limbs commonly involves those muscles innervated by the sciatic nerve (L6-S1 nerve roots, but L7 and S1 provide major contribution), especially flexor and extensor muscles of the hock. The patellar reflex may be hyperreflexic due to loss of antagonism from the flexor muscles (pseudo-hyperreflexia). The cranial tibial and gastrocnemius reflexes may be hyporeflexic. The flexor withdrawal reflexes often are reduced in the stifle and hock joints. Less commonly, the pudendal and coccygeal nerves may be involved. The pudendal nerve (S1-2 and S3) innervates the perineal region including the external anal and urethral sphincters. The coccygeal nerves innervate the tail. Decreased tail tone is assessed upon palpation and inability to wag.
Other clinical signs more common with chronic disease include fecal and urinary incontinence. If micturition dysfunction is suspected, it is important to closely evaluate sensory perception of the perineal region and the anal reflex. A digital rectal examination will assess for decreased rectal tone and further assess the urethra and prostate.
Diagnosis
A diagnosis of lumbosacral syndrome is suspected from the neurologic examination. Definitive diagnosis of DLSS is difficult because not one test has 100% specificity and sensitivity causing false positives and negatives. Diagnostic procedures advocated to evaluate for DLSS include electrophysiology, survey radiography, myelography, epidurography, and computed tomographic (CT) and magnetic resonance (MR) imaging. Electromyography is used to evaluate for spontaneous activity (fibrillation and positive sharp waves) in the following muscle groups: limbs, lumbosacral and caudal paraspinal, external anal sphincter and pelvic diaphragm muscles (Oliver 1978; Sisson 1989). Abnormal electrodiagnostic findings support pathology; however, negative findings do not rule out lumbosacral pathology. Abnormalities can be evaluated for in tibial-sciatic nerve conduction studies and somatosensory evoked potentials (Meij 2006).
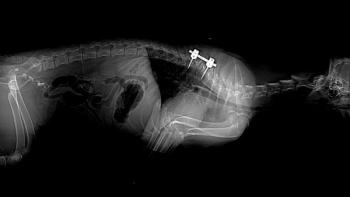
Survey radiography is useful to rule out other causes of cauda equina syndrome, e.g. diskospondylitis. Abnormal findings that have been evaluated in dogs with DLSS include: osteochondrosis of the sacral end plate (Lang 1992; Hanna 2001), transitional vertebrae (Morgan 1993), spondylosis (Wright 1980), subluxation, sclerosis of the end plates, and bony proliferation of the articular processes. Stress radiography (extension and flexion) has been used to identify underlying instability of the LS junction. Controversy still exists as to the significance of these radiographic signs. Radiographic studies of LS angle, range of motion and vertebral alignment fail to show association between classic radiographic and clinical signs (Schmid 1993). A recent retrospective study of spondylosis deformans found higher rates of spondylosis at sites with type II disk protrusions (Levine 2006). However, other studies have shown that spondylosis deformans is not consistently associated with clinical signs of DLSS (Scharf 2003, Steffan 2007). Dogs with evidence of transitional vertebrae (Morgan 1993, Fluckiger 2006) or osteochondrosis (Lang 1992) on survey radiographs have increased risk for development of DLSS. However, results of a recent study suggested that specific radiographic abnormalities may be of limited use to identify working dogs at risk for developing DLSS (Steffen 2007).
Myelography is a contrast procedure mainly used to rule out other causes of compressive myelopathies cranial to L4. Although it may be used to assess the lumbosacral region (Lang 1988), due to early termination of the dural sac, the contrast column may not cross the LS junction in large breed dogs. Epidurography is a contrast procedure used to evaluate for dynamic and compressive within the epidural space of the LS region (Selcer 1988).
CT and MR imaging have the advantage of better bone and soft tissue resolution. Transverse, dorsal and sagittal planes provide determination of lesion extent. The articular processes, intervertebral disc and foramina are evaluated. The procedure should be performed prior to injection of any contrast medium into the canal or subarachnoid space. Abnormalities detected by CT include: loss of epidural fat, increased soft tissue opacity in the intervertebral foramen, bulging of the intervertebral disc, thecal sac displacement, spondylosis, narrowed vertebral canal, thickened articular processes, and osteophyte formation of articular processes and in the intervertebral foramen (Jones 1996 and 1999). CT tends to have a higher sensitivity for diagnosis of degenerative LSS than epidurography and discography. MR imaging is superior to CT with regard to soft tissue definition (de Haan 1993). The spinal cord, cerebrospinal fluid, intervertebral discs, ligaments and nerve roots can be directly visualized. MR imaging can provide early recognition of intervertebral disc degeneration. Disadvantages include longer imaging times, expense and less readily available. Furthermore, imaging findings based on CT and MR may be disparate when compared to severity of clinical signs and predicting surgical outcome (Jones 2000, Mayhew 2002).
Treatment
Conservative
Degenerative LSS is managed conservatively or surgically. Indications for conservative management include the first episode of clinical signs or when the pain is intermittent. Management consists of strict confinement for 8 to 14 weeks, anti-inflammatory medication using low dose prednisone or NSAIDs and weight loss. The recovery rate with conservative management is between 24 and 50% (Ness 1994). Signs often recur when exercise is resumed.
Surgical
Indications for surgical management include failure of conservative management, severe pain and severe neurologic deficits (in particular incontinence). Choices of surgical procedures include dorsal decompression, diskectomy, facetectomy, foraminotomy, and fixation-fusion. Surgical decompression is the most common treatment (Chambers 1988). Dorsal laminectomy allows decompression and visualization of the cauda equina. The nerve roots can be retracted laterally for visualization of the disc for annular fenestration. A foraminotomy can be performed using a bone curette or pneumatic drill. It is important to salvage the articular processes because sacrifice of these structures may destabilize the LS joint. Recently, a lateral approach has been developed for foraminotomy (Godde 2007). Dorsal laminectomy provides relief of pain in most dogs. Short term outcome success using a decompressive laminectomy procedure ranges between 41-78% (Danielsson 1999; Chambers 1988; De Risio 2001). Recent attention also has been paid to biomechanical implications associated decompressive procedures. Studies have shown that laminectomy alone does not cause instability, but the addition of diskectomy reduces stiffness by 33% and the further addition of facetectomy is likely to have devastating consequences to stability of the LS region (Smith 2004).
Dorsal stabilization procedures include distraction-fusion, fusion, lag screw of facets and Kirschner techniques (Auger 2000; Slocum 1986 and 1989). The purpose of the distraction-fusion technique is to enlarge the collapsed disc space and foraminae. The lag screw technique has the potential to distract but may further weaken and fracture the articular processes. If there is potential for instability, the author prefers placement of threaded pins into the vertebral bodies of L7 and the sacrum and using polymethylmethacrylate to bridge the pins (Sturges 2003; Weh 2007). Long term outcome still remains to be determined with this procedure. Pedicle screw-rod fixation technique also has been described to stabilize the L7-S1 junction in dogs (Meij 2007; Carpenter 2006).
A critical aspect to postoperative care is strict cage confinement for 8-12 weeks and a gradual return to fitness. Additionally, bladder management involves proper monitoring of bladder emptying to avoid urinary tract infection.
Prognosis
Prognosis is fair to good if clinical signs resolve with surgery. Recurrence rates for degenerative LSS vary between 3% and 18% in the working dog (De Risio 2001; Danielsson 1999). Working dogs with DLSS can have a good prognosis with surgical decompression alone if they are younger dogs with mild clinical signs at time of diagnosis; however, recurrence may be seen (Linn 2003). Dogs with severe neurologic deficits, and urinary and fecal incontinence for more than a few weeks prior to surgery have a guarded to poor prognosis (De Risio 2001). Other reported recurrence rates have varied with relation of activity level of the dog with the active working dog having higher level of recurrence (Janssens 2000). Current knowledge of long-term outcomes for treatment of DLSS is limited to anecdotal evidence and retrospective studies with need for further studies evaluating specific outcome measures. Recently force plate analysis and questionnaire were used to provide outcome assessment for dogs treated with decompressive surgery (Suwankong 2007). Findings revealed that propulsive forces of pelvic limbs in dogs with DLSS are impaired and partially restored by decompressive surgery.
Selected references available upon request
Parts of these proceedings were adapted from – Coates JR. Tail, anal and bladder dysfunction. In: Platt S, Olby N (eds): BSAVA Manual of Canine and Feline Neurology 3rd edition. BSAVA., pp. 302-319, 2004.
Also presented at the 145th AVMA Annual Convention, New Orleans, LA. July 19-22, 2008.
Newsletter
From exam room tips to practice management insights, get trusted veterinary news delivered straight to your inbox—subscribe to dvm360.