The effect of USP Regulations 795, 797, and 800 on veterinary hospitals
Updates to safety measures for handling hazardous drugs
Oncology treatment area with a chemotherapy administration room and a separated animal housing area in the background. (Image courtesy of Tim Murphy | Photo Imagery)

Veterinary medical professionals working in oncology or reproductive-related services are likely aware of the United States Pharmacopeia (USP) General Chapter <800> Hazardous Drugs – Handling in Healthcare Settings. But, with the official acceptance of chapters <795> and <797> as of November 1, 2023, everyone within the veterinary industry should now be aware of these recent, important updates from the USP and how they will affect your team in compounding drugs for sterile and nonsterile preparations.
USP is a compendium of drug information that publishes standards for both humans and animals. In March 2020, the USP appeals panel remanded chapters 795 and 797 to the Compounding Expert Committee (CMP EC). These chapters deal directly with nonsterile and sterile pharmaceutical compounding and are designed to help protect the health and safety of both veterinary staff and animals. Some of the themes of the review included:
- A risk-based approach to assigning beyond-use dates (BUDs).
- Physical and chemical stability considerations.
- Sterility assurance for these pharmaceuticals.
- Operational and cost implications.
- Balancing patient access to cost-effective compounded nonsterile preparations (CNSPs) with rigorous quality standards.
- Implications for regulatory oversight and enforcement of these USP standards.1
The purpose of the revisions to 795 and 797 was to align with each other and with chapter 800, clarify common misconceptions, respond to input from public comments, and review the most up-to-date science and best practices.1 As of November 1, 2023, veterinary facilities are expected to meet the requirements of these standards. With the acceptance of chapters 795 and 797, General Chapter 800 will be enforceable. Chapter 800’s intent is to minimize risk to patients and the veterinary team when handling hazardous drugs.
It should be noted that the USP has no role in enforcing these standards. Enforcement is the responsibility of the FDA and other government bodies. For the veterinary industry, the task of enforcement falls on boards of pharmacy and/or state veterinary boards. The following is an overview of the most relevant changes to 795 and 797 because of the revisions.
General Chapter <795> Pharmaceutical Compounding – Nonsterile Preparations
Chapter 4 – Buildings and facilities
- A requirement was added for a designated area for nonsterile compounding.
- Other activities must not occur in the compounding area at the same time as compounding.
- This area must be well lit and must be maintained in a clean, orderly, sanitary condition and in a good state of repair.
- There was a flooring finish revision related to carpeting that changed the wording from must not to should not to accommodate those facilities that carry out infrequent nonsterile compounding.
Chapter 10 – Establishing BUDs
This is where a lot of time was spent on revisions and responses to public comments.
- The major points of this chapter have remained consistent.
- Section 10.1 – Terminology now differentiates BUDs from expiration dates.
- Expiration dates now apply only to conventionally manufactured drug products.
- BUDs apply to CNSPs and are calculated in terms of hours, days, or months.
- A new table was added to clarify BUD limits by type of preparation in the absence of a USP-NF compounded preparation monograph or CNSP-specific stability information.
- The concept of Water Activity (aw) was introduced to assess the susceptibility of a nonsterile preparation to microbial contamination and the potential for degradation from hydrolysis.
General Chapter <797> Pharmaceutical Compounding – Sterile Preparations
Removes provisions for the handling of hazardous drugs that are now only located in chapter 800.
Chapter 1 – Administration
- Provides the first reference to a designated person or persons to be responsible for ensuring the compounding operations within this chapter are performed per its rules.
- This also establishes the required qualifications for the identified personnel.
- Section 1.2 states that administration is out of the scope of the chapter.
- Section 1.5 – Categories
- The categorization of CSPs changed from microbial contamination risk levels (i.e., low-, medium-, and high-risk) to Category 1 and 2 CSPs. Additionally, Category 3 CSPs were added related to establishing BUDs.
- Category 1 and 2 CSPs are assigned a BUD based on certain risk factors including, the compounding method, whether sterility testing is performed, the starting ingredients used for the compounding, and the conditions under with the compound will be stored.
- Category 3 CSPs are assigned a BUD based on the compounding method and the storage conditions.
- Sections 1 and 15 provide information on single-dose containers.
- Per this section, a single-dose container cannot be used to prepare doses for more than 1 patient when compounding an immediate-use CSP.
- The definition of immediate use allows for up to 4 hours before administration begins.
- This section also includes clarification with updated CSP categories and how they relate to the hospital’s Primary Engineering Controls (PEC), as well as requirements for the location of clean and dirty sides of the facility’s anteroom.
So now that these chapters are official, how do they apply to you?
When working with our clients to renovate or design a safe space for handling compounded drugs, one of the first questions we get asked is, “What is the definition of compounding?”
USP has issued some great resources to help answer this question. In their FAQ Documents,2,3 they define compounding as the following, “combining, admixing, diluting, pooling, reconstituting other than as provided in the manufacturer’s labeling, or otherwise altering a drug product or bulk drug substance to create a sterile or nonsterile preparation.” Frequently people assume that what they are doing with certain drugs doesn’t fall under the definition of compounding and that these USP chapters don’t apply to them. This is often not the case. To limit, or possibly eliminate the amount of compounding done within your facility, in some situations, it is possible to change how drugs are received, or how they are handled.
Sterile versus nonsterile
A sterile compounding room in the Oncology department of a veterinary hospital designed to meet USP standards.
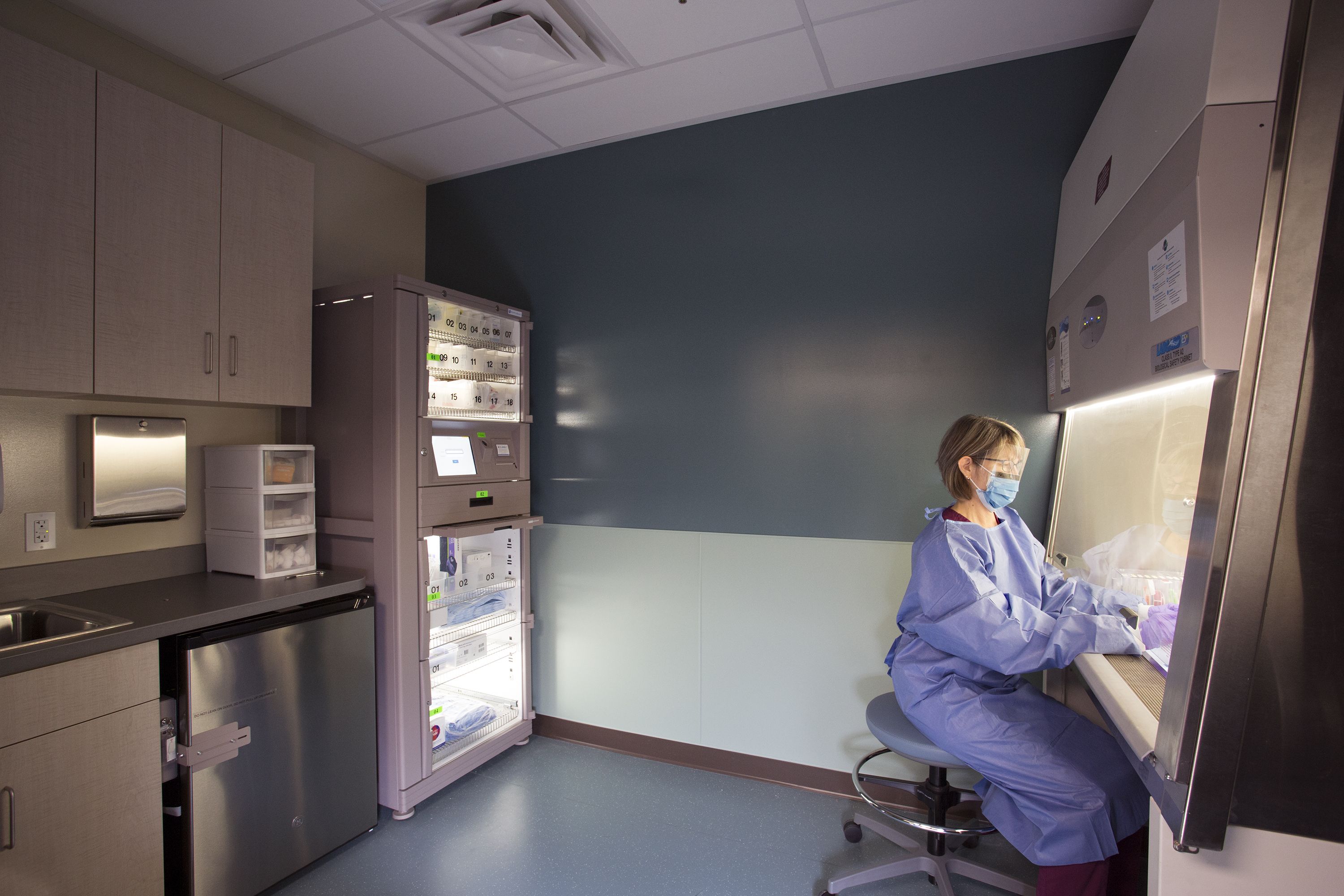
The next question we hear most frequently is “How do you differentiate between sterile and nonsterile?” In general, preparations designed to be administered to a body space with no contact with the environment outside of the body, such as the bladder cavity or peritoneal cavity, are typically required to be sterile. “Medications that are required to be sterile include those administered through injection, intravenous infusion (IV), intraocular (injection in the eye) or intrathecal (injection into the spine).4' Additionally, pharmaceuticals meant for the eye and aqueous inhalation solutions and suspensions must be sterile.
Otic preparations are not required to be sterile unless they are administered to a patient at the site of a perforated eardrum. Examples of nonsterile preparations would be irrigations for the mouth or sinus cavity. Withdrawing a dose from a container of a conventionally manufactured sterile drug or spiking an IV bag, without any additional manipulation, for immediate administration to a patient is NOT considered to be compounding. This is simply administration. If, however, the dose is mixed with another product, it would then be considered compounded and subject to 795 or 797.
Beyond use dates
BUDs are defined as the date, or date and hour, after which a CSP or CNSP cannot be used, stored, or transported. This is determined by the date and time the preparation is compounded. By adhering to BUDs, the risk posed to patients through physical or chemical degradation, microbial contamination, proliferation, and any effect on the integrity of the container closure system is decreased.
So where do you start in understanding all of this? The first step is to read the official USP chapters. While not a quick read, this is the best starting place. In addition, take notes and read the FAQs and other informational pieces that you can readily access on the USP’s website.
The USP compounding chapters require that a designated person or persons be assigned responsibility and accountability for oversight at each facility. This person or persons needs to be trained and qualified as an expert in the subject matter. There is specific information about these requirements in the various USP compounding chapters. This person should be the one to begin reviewing the chapters and putting together your risk assessment.
I recommend starting with a detailed drug questionnaire, or drug risk assessment to help understand your drug usage and how you are handling these drugs within your facility. This will help you assess your risk and determine the operational and facility needs to enable you to meet the standards. This questionnaire should include types of drugs used, frequency of use, administration type (IV, IM, etc.), as well as the BUDs. You will want to use the most up-to-date list of hazardous drugs when compiling this assessment. NIOSH, the CDC’s National Institute for Occupational Safety and Health current list.5
Once you assess your risk, you can then focus on making decisions with your team on modifying how you receive, compound, and administer these drugs. The higher the risk, the more rules your team will need to follow to carry out operations in a safe manner for both your patients and staff. The Assessment of Risk (AoR) is also laid out in USP <800> as a systematic way for organizations to ensure safe practices.
Reference
- Open Forum Session - Revisions to USP General Chapter (795) Pharmaceutical Compounding - Nonsterile Preparations. United States Pharmacopeia. Presented: Virtually, November 8, 2022. https://uspevents.webex.com/recordingservice/sites/uspevents/recording/d83d929b419e103badb18e1b4c569c84/playback )
- <795>FAQS. United States Pharmacopeia. Accessed January 30, 2024. https://go.usp.org/USP_GC_795_FAQs
- <797>FAQS. United States Pharmacopeia. Accessed January 30, 2024. https://go.usp.org/USP_GC_795_FAQs
- Pharmaceutical compounding – sterile preparations. United States Pharmacopeia. Accessed January 30, 2024. https://www.usp.org/compounding/general-chapter-797
- NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings, 2016. (Supersedes 2014-138). U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 2016.
Newsletter
From exam room tips to practice management insights, get trusted veterinary news delivered straight to your inbox—subscribe to dvm360.