Treating cancer pain in dogs and cats
Recent publications, ongoing prospective studies, and better knowledge of the available therapeutic options should provide the necessary framework for appropriate pain management in cancer-bearing pets.
Pain frequently accompanies cancer in people and is also common in cancer-bearing dogs and cats. Pain negatively affects quality of life and important physiological functions, and eliminating it should be a priority. Whether cancer pain has been identified, is suspected, or is expected to occur in a cancer-bearing patient, efforts should be directed at treating and preventing it effectively. While pain in certain dogs and cats with cancer will be relieved with treatment directed toward the underlying cancer, others will require symptomatic analgesic therapy to improve their quality of life. A relative abundance of literature discusses the management of cancer pain in people.1-7 In contrast, there is a paucity of information specifically regarding the management of veterinary cancer pain. Recent publications, ongoing prospective studies, and better knowledge of the available therapeutic options should provide the necessary framework for appropriate pain management in cancer-bearing pets.8-14
METHODS OF PAIN RELIEF FOR CANCER PATIENTS
The most rational way to alleviate cancer pain, whenever possible, is to treat the underlying tumor. Always evaluate the possibility of surgically removing a resectable tumor or obtaining a remission by using radiation therapy for a radiosensitive tumor or chemotherapy for a chemosensitive cancer. While you are waiting for the definitive therapy to take effect and eliminate the source of pain, analgesic and supportive therapies are often required to improve a patient's quality of life. And occasionally, with resistant, refractory, recurring, or terminal cancers, only palliative therapy can truly benefit a patient.
The goal in managing dogs and cats with cancer is to control pain and improve a patient's overall quality of life by using traditional anticancer therapeutic modalities, various analgesic therapies, and supportive care. Before you treat for cancer pain, you must have a basic understanding of the pathophysiology of pain and of the methods used to recognize and evaluate pain in veterinary patients (see the article "Understanding and recognizing cancer pain in dogs and cats").
TREATMENT DIRECTED AT THE TUMOR
Surgery
Whenever possible, completely remove a painful tumor surgically. With an appropriate preemptive and multimodal analgesic program in place, including postoperative pain management, most patients with tumors causing pain will be more comfortable after radical surgeries. Surgery is the modality that can most rapidly eliminate the source of pain, and various surgical procedures can be performed, depending on the tumor's type, extent, and location. Examples of tumors best treated and possibly cured with surgery alone include canine oral tumors such as squamous cell carcinoma and fibrosarcoma, cutaneous mast cell tumors, mammary tumors, soft tissue sarcomas, splenic or hepatic tumors with painful capsular distention, nail bed tumors, synovial cell sarcoma causing bone lysis, and tumors of the ear canal.
Occasionally, patients will benefit from palliative surgeries to remove painful tumors, although the surgery itself may not positively affect the overall prognosis for survival. A classic example is a painful appendicular osteosarcoma in which metastatic disease ultimately determines the survival time. In this example, amputating the limb provides immediate relief to the patient, and adjuvant chemotherapy is administered to prolong survival and delay the growth of micrometastatic disease.
Surgically removing pulmonary metastatic lesions to provide relief from painful paraneoplastic hypertrophic osteopathy has been described. In a recent study in four dogs with osteosarcoma, pain relief from hypertrophic osteopathy was observed in all dogs less than 24 hours after metastasectomy.15 Peripheral and central neuroablative surgeries can be contemplated in select cases; these surgeries have proved valuable in people with terminal cancer and poorly responsive pain. Such salvage analgesic techniques are relatively novel and have yet to be described for the control of veterinary cancer pain.1-3
Radiation therapy
Radiation therapy is valuable in treating many veterinary cancers. Full-course fractionated protocols, also called curative-intent protocols, are used to treat radiosensitive tumors in which a clinical benefit and survival advantage can be expected from treatment. With fractionated protocols, a small dose, or fraction, is administered daily or every other day. Painful macroscopic tumors that are occasionally treated this way include sinonasal tumors (carcinomas, sarcomas, or lymphomas), certain oral tumors (ameloblastoma, squamous cell carcinoma), solitary osseous plasma cell tumors (Figures 1A & 1B), certain anatomical forms of lymphoma (mediastinal, central nervous system), and thyroid carcinomas.
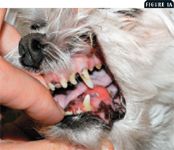
Figure 1A. The gross (1A) and radiographic appearance (1B) of a painful osteolytic mandibular lesion caused by a malignant plasma cell tumor in a 9-year-old Maltese. This dog, which had numerous other lesions (one other bone lesion on the contralateral maxilla and multiple skin nodules), received coarse fractions of radiation therapy, pamidronate infusions, systemic chemotherapy, and low-dose corticosteroids. These treatments produced marked clinical improvement.
For painful tumors that may not respond as well to fractionated protocols, a protocol that uses coarse fractions of radiation, often called palliative radiation therapy, can help alleviate pain in many patients. With coarse fractionated protocols, large fractions are generally administered once or twice a week for two to four weeks. Painful tumors treated in this fashion include canine oral malignant melanoma (complete responses observed in as many as 80%), feline oral squamous cell carcinoma, osteosarcoma (appendicular or axial, primary or metastatic), unresectable mast cell tumors, unresectable subcutaneous or intramuscular hemangiosarcoma, and bone metastases (various carcinomas).16-24
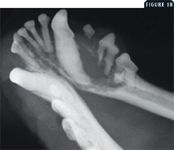
Figure 1B. The gross (1A) and radiographic appearance (1B) of a painful osteolytic mandibular lesion caused by a malignant plasma cell tumor in a 9-year-old Maltese. This dog, which had numerous other lesions (one other bone lesion on the contralateral maxilla and multiple skin nodules), received coarse fractions of radiation therapy, pamidronate infusions, systemic chemotherapy, and low-dose corticosteroids. These treatments produced marked clinical improvement.
Painful early side effects of radiation therapy, especially oral mucositis, acute moist dermatitis, colitis, and conjunctivitis, tend to be more commonly encountered with full-course (curative-intent) daily fractionated protocols. In all cases, preventing additional self-mutilation is essential, and Elizabethan collars should be used as needed. Oral mucositis can be symptomatically treated with oral rinse solutions (a weak tea solution, chlorhexidine rinse, or mixture of viscous lidocaine, liquid diphenhydramine, and magnesium hydroxide) and systemic nonsteroidal anti-inflammatory drugs (NSAIDs) with a weak opioid as needed. Signs of colitis or proctitis can be effectively relieved with a corticosteroid enema (Proctofoam HC—Schwarz Pharma Inc.) inserted once or twice a day. Patients with acute moist dermatitis may benefit from topical application of colloidal oatmeal (Aveeno Soothing Bath Treatment—Johnson & Johnson Consumer Companies Inc.), wheat extracts (Damor Saturation Cream—Damor America), or aloe gel extract.10,25 Avoid petroleum-based products as they may impair healing of irradiated tissues. In all cases, broad-spectrum antibiotics may help control opportunistic bacterial infection.
Chemotherapy
Systemic cytotoxic chemotherapy is used to treat chemosensitive tumors or cancers in patients in which a clinical remission can be obtained. While lymphoma is not perceived as a painful cancer, in certain anatomical locations such as the central nervous system or bone, lymphoma will result in pain. In addition, certain patients with hepatosplenic disease may occasionally have visceral pain from capsular distention. Multiple myeloma is often painful, with osteolysis occurring in multiple osseous sites, and can also result in pathologic fractures or compression of the meninges and spinal cord. Chemotherapy remains the mainstay of therapy for multiple myeloma, but other therapies can be concurrently administered in affected patients to alleviate pain rapidly, including radiation therapy and anti-osteoclastic drugs such as bisphosphonates. Many patients with painful mast cell tumors may benefit from systemic chemotherapy when their disease is unresectable or disseminated (see "Treatment options for canine cutaneous mast cell tumors" in the April 2005 issue). Painful carcinomatosis is occasionally treated with intracavitary chemotherapy. Finally, transmissible venereal tumors are found most typically on mucosal surfaces of the external genitalia and can cause pain and discomfort in sexually intact dogs. These tumors are chemosensitive to monotherapy with the tubulin-binding agent vincristine.
Multimodality therapy
While surgery, radiation therapy, or chemotherapy may be used alone for certain tumors, they are frequently combined to achieve better and longer tumor control, translating into prolonged and more complete pain alleviation.19,24,26,27 Examples of cancer best treated with multimodality therapy include feline vaccine-associated soft tissue sarcomas, canine apocrine gland anal sac adenocarcinoma, high-grade canine mast cell tumors and soft tissue sarcomas, and certain oral tumors.
ANALGESIC DRUGS
The above treatments are directed at the tumor itself. In addition, many analgesic drugs can be administered to treat pain in general in patients with cancer.
NSAIDs
By far, NSAIDs are the drugs most veterinarians will initially use to control various types of pain in companion animals. NSAIDs' main mechanism of action is through inhibiting cyclooxygenases (COXs). COX-2 is the main target of recently developed selective inhibitors because of its role in inflammation and pain; it is an inducible enzyme that leads to the production of prostaglandin E2 and other eicosanoids from arachidonic acid. COX-1 has often been referred to as the good COX since it is the isoform responsible for homeostasis (gastrointestinal mucosal protection, renal blood flow, platelet aggregation), and COX-2 has been called the bad COX because of its involvement with pain, inflammation, fever, ischemia, and cancer. It is now apparent that COX-2 may also carry important physiological functions, with basal expression levels demonstrated in tissues such as the ovaries, uterus, brain, spinal cord, kidney, cartilage, bone, and gut.2
As a class, NSAIDs can be helpful in treating cancer pain for numerous reasons. Prostaglandins play an important role in peripheral sensitization of certain nociceptors, leading to a state of hyperalgesia, an exaggerated response to a mildly noxious stimulus, or allodynia, a painful response after a non-noxious stimulus.2,9 The prostaglandins can also activate certain sodium channels in the dorsal horn of the spinal cord and cause changes resulting in central sensitization and establishment of a state of chronic pain. Peripheral and central sensitization are common in chronic cancer pain.2,9 Hence, it seems rational to use NSAIDs, in combination with other drugs, to treat conditions of moderate to severe chronic cancer pain. (For a three-step analgesic ladder for controlling mild, moderate, and severe pain proposed by the World Health Organization, see the article "Understanding and recognizing cancer pain in dogs and cats".)
NSAIDs are effective to some degree in people for treating moderate to severe pain from bone metastases, compression of muscles and tendons, and distention of the peritoneum or pleura.2 Veterinary patients with cancer pain also appear to benefit from the use of NSAIDs, and they are often used alone for mild pain or in combination with opioid drugs and adjuvant analgesics for moderate and severe pain. When used for cancer pain, the various veterinary-approved NSAIDs appear to be equianalgesic.11 The clinical response and toxicity profile may vary among patients receiving a given drug. If a patient fails to benefit from a given NSAID for pain control or suffers untoward side effects, it is reasonable to try another drug, ideally after a washout period of seven to 10 days, although no guidelines have been established.
Another rationale to use NSAIDs for cancer pain stems from information gathered in the last decade regarding the potential anticancer and chemopreventive effects of these drugs in human and veterinary patients. Overexpression of COX-2 has been demonstrated to occur in many human and canine tumors, including carcinomas of the bladder, prostate, mammary gland, intestine, kidney, skin and oral cavity (squamous cell carcinoma), and nasal cavity, as well as in some malignant melanomas and osteosarcomas.28-38 This overexpression may lead to a decreased immune response against the cancer cells, decreased apoptosis (programmed cell death), increased angiogenesis, and increased proliferation. Therefore, using NSAIDs could have both analgesic and antitumor effects. Many studies in dogs have demonstrated tumor responses with NSAID monotherapy, and others have shown a possible additive effect when combined with standard chemotherapy agents.35,39-44
In the last 10 years, an ever-expanding number of veterinary NSAIDs have been approved, much to the benefit of our patients, especially dogs. In the North American market, these include drugs such as deracoxib (Deramaxx—Novartis Animal Health; United States), carprofen (Rimadyl—Pfizer Animal Health; United States and Canada), meloxicam (Metacam—Boehringer Ingelheim and Merial; United States and Canada), etodolac (Etogesic—Fort Dodge Animal Health; United States), tepoxalin (Zubrin—Schering-Plough Animal Health; United States), tolfenamic acid (Tolfedine—Vétoquinol; Canada), and ketoprofen (Anafen—Merial; Canada). Other drugs are occasionally used in companion animals and include aspirin (ArthriCare—Veterinary Products Laboratories) (approved for and primarily used in dogs), acetaminophen (dogs only, not veterinary approved), and piroxicam (dogs and cats, not veterinary approved). Table 1 provides NSAID dosages commonly used for treating chronic cancer pain [For clarity, all drugs listed in Table 1 will be referred to as NSAIDs in the text].

Table 1. NSAIDs Used to Treat Cancer Pain in Dogs and Cats
It is recommended to perform a complete blood cell count, serum chemistry profile, and urinalysis to evaluate renal and liver function before administering NSAIDs long-term in veterinary patients. It is especially important to measure serum urea nitrogen and creatinine concentrations, urine specific gravity, liver enzyme activities (alkaline phosphatase and alanine transaminase), and, occasionally, serum bile acid concentrations (preprandial and postprandial) when liver enzyme activities are elevated. Obtain baseline values, followed by a recheck after two to four weeks and then periodic reassessment every two to four months with long-term therapy. Instruct owners to look for specific signs such as melena, vomiting, lethargy, decreased appetite, depression, yellow discoloration of mucous membranes and sclera, and altered water intake and urine output. The most common side effects of all NSAIDs remain gastrointestinal irritation and nephrotoxicosis. Hepatotoxicosis can also occur with acetaminophen or because of idiosyncratic reactions to NSAIDs. Antithrombotic effects can also occur with aspirin.
Few NSAIDs are approved for cats, and their use has been rendered difficult because of marked differences in metabolism when compared with dogs. Species differences in the glucuronidation pathways play an important role in the metabolism of many such drugs and account for the prolonged half-life of NSAIDs in cats when compared with that in dogs.45 The NSAIDs labeled for use in cats in North America currently include meloxicam, tolfenamic acid, and ketoprofen (Table 1). Although drugs such as carprofen and piroxicam are not approved in cats, studies have been performed, and relatively safe dosages and dosing intervals have been reported.45-47 Using the lowest effective dose and avoiding their use in cats with altered renal function are the basics of safe NSAID administration in cats.11 Acetaminophen is extremely toxic in cats and should never be used.
Opioids
Opioids are considered the mainstay of cancer pain therapy in human oncology, and no good reason exists for it to be any different in veterinary oncology (Table 2).1-3 One reason opioids are so helpful is that their dosage can be gradually and safely increased as needed to provide the desired level of analgesia. Side effects can be more important at higher dosages but are relatively predictable and tolerable, except for dysphoria in cats and Nordic canine breeds. Other main side effects include sedation, constipation, bradycardia, respiratory depression, panting, mydriasis (cats), cough suppression, altered laryngeal reflexes, vomiting, and histamine release (mostly morphine, especially with rapid intravenous dose).1-3,9 These side effects are encountered more commonly at higher dosages and with long-term use, and some can be treated symptomatically (constipation, vomiting).2,10,48,49 Opioids are used mostly for moderate to severe pain, and their effects are potentiated when used concurrently with other analgesics, including NSAIDs. The three main types of opioid receptors are mu, delta, and kappa, and they are located primarily in the superficial dorsal horn. The clinically used and best analgesic opioids have mu-receptor agonistic activity.2
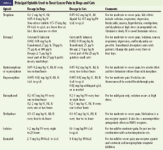
Table 2. Principal Opioids Used to Treat Cancer Pain in Dogs and Cats
The standard opioid remains morphine, a pure mu-receptor agonist. Morphine is relatively inexpensive, has predictable and tolerable side effects, and can be administered by various routes (subcutaneous, intramuscular, intravenous, oral, epidural). Other mu agonists useful in treating cancer pain include fentanyl (transdermal, intravenous infusion), hydromorphone, and oxymorphone. The use of transdermal fentanyl in companion animals has been recently reviewed (Figure 2).50 The partial agonist buprenorphine has a long duration of action and can be useful, especially with the effective transmucosal (mouth) route in cats, for treating breakthrough pain.9,11,14,45 Butorphanol, a mixed mu antagonist and kappa agonist, is not considered adequate for treating cancer pain, especially when pain is moderate to severe.2,3 In addition to butorphanol's lesser analgesic potency when compared with pure mu agonists, its duration of analgesia is short (20 to 90 minutes) when compared with the duration of sedation (hours), often leading to a false interpretation of comfort in sedated but painful patients.9,51-53 An opioid that is increasingly used for cancer pain in people is methadone.1-3 This interesting opioid not only is a mu-receptor agonist but also produces additional analgesic effects through noncompetitive antagonism of the N-methyl-D-aspartate (NMDA) receptors.1-3,9 Methadone has recently been described for treating pain in companion animals.9,14,45
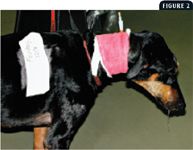
Figure 2. This 8-year-old Doberman pinscher is being treated with full-course radiation therapy for a nasal tumor and has early radiation side effects (mucositis). An esophageal feeding tube was placed for enteral nutrition, and the dog was also treated with a transdermal fentanyl patch, an oral NSAID, and a mouthwash solution (viscous lidocaine, magnesium aluminum hydroxide, diphenhydramine) given once or twice a day.
Weaker opioids, such as codeine and tramadol, can be useful for moderate cancer pain. Tramadol has been around for over 15 years and has received attention in recent years regarding its use for chronic pain, both in people and companion animals. Tramadol is considered a good analgesic for moderate cancer pain in people and is better tolerated than equianalgesic true opioids.2,3,7,54,55 Recent pharmacokinetic and pharmacodynamic studies in dogs, in addition to the growing body of anecdotal evidence of analgesic efficacy by various investigators, suggest that tramadol may be safely and effectively used for various pain conditions in dogs.56-59 Still, few reports exist on the efficacy of tramadol for treating pain in dogs or cats, though studies are ongoing. Tramadol is a mu-receptor agonist and also has serotonin and norepinephrine reuptake inhibition effects.2 Combining it with other analgesics, including NSAIDs, provides better analgesia.
Alpha2 agonists
The main alpha2 agonists are medetomidine and xylazine, which are useful in the context of multimodal and preemptive analgesia. Their analgesic effects are especially pronounced when combined with other agents such as opioids and ketamine.9,14 Medetomidine is often used in combination with opioids to provide adequate analgesia for moderately to severely painful procedures and is known to have an opioid-sparing effect. Alpha2 agonists may cause bradycardia secondary to increased vagal tone and are contraindicated in patients negatively affected by a decreased cardiac output and increased afterload.9,14 They may also cause transient hypertension.
Currently, medetomidine is most commonly used as a preanesthetic or for short procedures requiring mild to deep sedation. It is most often combined with an opioid, and we recommend a dose of 1 to 6 µg/kg given intramuscularly or intravenously in dogs and a dose of 2.5 to 10 µg/kg intramuscularly or intravenously in cats. The lower end of the dose range is used for intravenous administration.
Adjuvant drugs
Several classes of drugs can be used as adjuvant therapy—local anesthetics, NMDA antagonists, anticonvulsants, tricyclic antidepressants, aminobisphosphonates, and corticosteroids (Table 3).

Table 3. Adjuvant Analgesic Drugs Used to Treat Cancer Pain in Dogs and Cats
Local anesthetics
Local anesthetics are valuable in many situations, and, being often used for local or regional blocks, their intravenous, oral, or transdermal application can also be helpful in certain conditions.4,7,9,14 A good local or regional block may help provide adequate pain control and permit much lower doses of systemic drugs. The systemic administration of certain sodium channel blockers, such as intravenous lidocaine and oral mexiletine, to potentiate analgesia from other drugs, is becoming more common in treating cancer pain in people.2,4,7,14 The systemic administration of local anesthetics for analgesia in dogs and cats is relatively recent and may increase in the near future.
NMDA antagonists
This class of analgesics includes drugs such as ketamine, tiletamine, amantadine, and dextromethorphan. Ketamine has been in use for many years as a dissociative agent, but its NMDA-receptor antagonistic effect is now considered an important part of its central analgesic effect.9 It is especially useful when combined with other agents and permits adequate analgesia with lower doses of opioids (sparing effect) and lower side effects. It can be effective for intraoperative and postoperative analgesia when used at a microdose in a continuous-rate infusion combined with fentanyl or morphine. Amantadine was initially developed and used as an antiviral agent against influenza in people and is available as an oral preparation. It also has NMDA-receptor antagonistic activity, and its use in people as an analgesic for chronic cancer pain has recently increased.2-4 No studies have evaluated the use of amantadine for pain treatment in dogs and cats, but investigations are ongoing.9,11,14
Anticonvulsants
Anticonvulsants appear to be useful in the management of neuropathic pain and chronic pain with central sensitization. The anticonvulsant most commonly discussed is gabapentin, a structural analogue of gamma-aminobutyric acid (GABA), which appears to provide analgesia by modulating both sodium and calcium channels affecting NMDA and perhaps other receptors.2,4,7,9,11,14 While the use of gabapentin for veterinary cancer pain is poorly described in the literature, it is known to be well-tolerated, highly bioavailable, and rapidly metabolized in dogs, and it appears to work best when used in combination with other analgesic agents, such as NSAIDs.9,11,14,60 Currently, only anecdotal evidence supports the use of gabapentin for chronic or neuropathic pain relief.
Tricyclic antidepressants
Tricyclic antidepressants have analgesic effects for both nonmalignant pain conditions and for chronic cancer pain in people.1-4,7 This class of drugs includes amitriptyline and the veterinary-approved clomipramine, among others. While the use of tricyclic antidepressants as adjuvant analgesic agents is still poorly described in veterinary medicine, they appear to be best suited for combination therapy with other classes of analgesic drugs.1-4,7,9,11,14,61 Their analgesic effects are thought to be attributed to actions on endogenous monoaminergic pain modulating systems, especially those using norepinephrine and serotonin.2
Aminobisphosphonates
Synthetic analogues of inorganic pyrophosphate, bisphosphonates are a class of drugs that, by specifically binding to sites of active bone turnover and inhibiting osteoclasts, can help manage malignant bone pain.1-4,62,63 By directly causing osteoclast apoptosis, bisphosphonates are especially useful to treat painful osteolytic bone lesions. The more potent intravenous aminobisphosphonates, including pamidronate and zoledronate, are preferred for osteolytic bone pain from metastatic carcinoma and multiple myeloma in people, and ongoing studies are evaluating their use in dogs and cats with primary and metastatic bone cancer (Figures 1A & 1B).62,63
Corticosteroids
Corticosteroids have a mild analgesic effect and can be occasionally considered for cancer pain, especially when inflammation participates in the ongoing nociceptive stimuli.1,2,4,7,10,11,14 They should not be used concurrently with NSAIDs.

Figure 3. This 13-year-old mixed-breed dog receives acupuncture treatments for osteoarthritis and back pain initially triggered by lytic lesions from multiple myeloma that is in prolonged complete remission following systemic chemotherapy.
Complementary therapies and rehabilitation medicine
More and more, complementary therapies are being introduced in the therapeutic regimen to treat cancer pain in people and may be applicable in dogs and cats.1,2,64,65 Such complementary therapies include acupuncture (Figure 3), massage, stretch and manipulation, hydrotherapy (Figure 4), play therapy, superficial heat and cold application, percutaneous electrical stimulation, transcutaneous electrical nerve stimulation, laser therapy, ultrasound, and pulsed magnetic field therapy.1,2,9,14,64,65 While their specific use for veterinary cancer pain has not been described in the literature, their increasing availability, anecdotal reported efficacy, and excellent tolerability profile make them an attractive addition to the therapeutic options in that setting.

Figure 4. An adult German shepherd receiving hydrotherapy on an underwater treadmill. (Photograph courtesy of Dr. Dianne Dunning.)
Combination therapy and drug interactions
A multimodal approach is best suited for treating and alleviating cancer pain, often combining two or more analgesics for moderate-to-severe, refractory pain. Use caution when treating geriatric patients with a variety of agents, and introduce new drugs one at a time, in a sequential manner.4 When side effects are encountered or if a drug is used at its maximum safe dose without benefit to a patient, its use should be discontinued.4 Carefully evaluate the possibility of pharmacokinetic or pharmacodynamic drug interactions whenever multiple drugs are used concurrently.
Louis-Philippe de Lorimier, DVM
Timothy M. Fan, DVM, DACVIM (internal medicine, oncology)
Department of Veterinary Clinical Medicine
College of Veterinary Medicine
University of Illinois
Urbana, IL 61802
REFERENCES
1. Foley KM. Management of cancer pain. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: principles and practice of oncology. 6th ed. Philadelphia, Pa: Lippincott Williams & Wilkins, 2001;2977-3011.
2. Bruera ED, Portenoy RK. Cancer pain: assessment and management. Cambridge, UK: Cambridge University Press, 2003.
3. Slavin KV, Tesoro EP, Mucksavage JJ. The treatment of cancer pain. Drugs Today 2004;40:235-245.
4. Lussier D, Huskey AG, Portenoy RK. Adjuvant analgesics in cancer pain management. Oncologist 2004;9:571-591.
5. Patt RB, Lang SS. The complete guide to relieving cancer pain and suffering. New York, NY: Oxford University Press, 2004.
6. Collective work. American Cancer Society's guide to pain control: understanding and managing cancer pain. Atlanta, Ga: American Cancer Society Health Promotions, 2004.
7. Fine PG, Miaskowski C, Paice JA. Meeting the challenges in cancer pain management. J Support Oncol 2004;2(6 suppl 2):5-22.
8. Gaynor JS. Pain management for the oncology patient. In: Withrow SJ, MacEwen EG, eds. Small animal clinical oncology. 3rd ed. Philadelphia, Pa: WB Saunders, 2001;219-232.
9. Gaynor JS, Muir WW. Handbook of veterinary pain management. St. Louis, Mo: Mosby, 2002.
10. Kyles AE, Ruslander D. Chronic pain: osteoarthritis and cancer. Semin Vet Med Surg (Small Anim) 1997;12:122-132.
11. Lascelles BD. Relief of chronic cancer pain. In: Dobson J, Lascelles BD, eds. BSAVA manual of oncology. 2nd ed. Cheltenham, UK: BSAVA Publications, 2003;137-151.
12. Lester P, Gaynor JS. Management of cancer pain. Vet Clin North Am Small Anim Pract 2000;30:951-966.
13. O'Brien MG. Cancer pain management: a review of recent considerations and advancements. Vet Cancer Soc Newsletter 1998;22:1-6.
14. Tranquilli WJ, Grimm KA, Lamont LA. Pain management for the small animal practitioner. 2nd ed. Jackson, Wyo: Teton NewMedia, 2004.
15. Liptak JM, Monnet E, Dernell WS, et al. Pulmonary metastatectomy in the management of four dogs with hypertrophic osteopathy. Vet Comp Oncol 2004;2:1-12.
16. Bateman KE, Catton PA, Pennock PW, et al. 0-7-21 radiation therapy for the palliation of advanced cancer in dogs. J Vet Intern Med 1994;8:394-399.
17. Green EM, Adams WM, Forrest LJ. Four fraction palliative radiotherapy for osteosarcoma in 24 dogs. J Am Anim Hosp Assoc 2002;38:445-451.
18. McEntee MC. Radiation therapy in the management of bone tumors. Vet Clin North Am Small Anim Pract 1997;27:131-138.
19. Ramirez O 3rd, Dodge RK, Page RL, et al. Palliative radiotherapy of appendicular osteosarcoma in 95 dogs. Vet Radiol Ultrasound 1999;40:517-522.
20. Siegel S, Cronin KL. Palliative radiotherapy. Vet Clin North Am Small Anim Pract 1997;27:149-155.
21. Thrall DE, LaRue SM. Palliative radiation therapy. Semin Vet Med Surg (Small Anim) 1995;10:205-208.
22. Jones PD, de Lorimier LP, Kitchell BE, et al. Gemcitabine as a radiosensitizer for nonresectable feline oral squamous cell carcinoma. J Am Anim Hosp Assoc 2003;39:463-467.
23. Bateman KE, Catton PA, Pennock PW, et al. 0-7-21 radiation therapy for the treatment of canine oral melanoma. J Vet Intern Med 1994;8:267-272.
24. Dobson J, Cohen S, Gould S. Treatment of canine mast cell tumours with prednisolone and radiotherapy. Vet Comp Oncol 2004;2:132-141.
25. LaRue SM, Gillette EL. Radiation therapy. In: Withrow SJ, MacEwen EG, eds. Small animal clinical oncology. 3rd ed. Philadelphia, Pa: WB Saunders, 2001;119-137.
26. Freeman KP, Hahn KA, Harris FD, et al. Treatment of dogs with oral melanoma by hypofractionated radiation therapy and platinum-based chemotherapy (1987-1997). J Vet Intern Med 2003;17:96-101.
27. Klein MK. Multimodality therapy for head and neck cancer. Vet Clin North Am Small Anim Pract 2003;33:615-628.
28. Doré M, Lanthier I, Sirois J. Cyclooxygenase-2 expression in canine mammary tumors. Vet Pathol 2003;40:207-212.
29. Mohammed SI, Khan KN, Sellers RS, et al. Expression of cyclooxygenase-1 and 2 in naturally-occurring canine cancer. Prostaglandins Leukot Essent Fatty Acids 2004;70:479-483.
30. Kleiter M, Malarkey DE, Ruslander DE, et al. Expression of cyclooxygenase-2 in canine epithelial nasal tumors. Vet Radiol Ultrasound 2004;45:255-260.
31. Mullins MN, Lana SE, Dernell WS, et al. Cyclooxygenase-2 expression in canine appendicular osteosarcomas. J Vet Intern Med 2004;18:859-865.
32. Khan KN, Stanfield KM, Trajkovic D, et al. Expression of cyclooxygenase-2 in canine renal cell carcinoma. Vet Pathol 2001;38:116-119.
33. Khan KN, Knapp DW, Denicola DB, et al. Expression of cyclooxygenase-2 in transitional cell carcinoma of the urinary bladder in dogs. Am J Vet Res 2000;61:478-481.
34. Tremblay C, Doré M, Bochsler PN, et al. Induction of prostaglandin G/H synthase-2 in a canine model of spontaneous prostatic adenocarcinoma. J Natl Cancer Inst 1999;91:1398-1403.
35. Sorenmo KU, Goldschmidt MH, Shofer FS, et al. Evaluation of cyclooxygenase-1 and cyclooxygenase-2 expression and the effect of cyclooxygenase inhibitors in canine prostatic carcinoma. Vet Comp Oncol 2004;2:13-23.
36. Pestili de Almeida EM, Piché C, Sirois J, et al. Expression of cyclo-oxygenase-2 in naturally occurring squamous cell carcinomas in dogs. J Histochem Cytochem 2001;49:867-875.
37. McEntee MF, Cates JM, Neilsen N. Cyclooxygenase-2 expression in spontaneous intestinal neoplasia of domestic dogs. Vet Pathol 2002;39:428-436.
38. Borzacchiello G, Paciello O, Papparella S. Expression of cyclooxygenase-1 and -2 in canine nasal carcinomas. J Comp Pathol 2004;131:70-76.
39. Mohammed SI, Craig BA, Mutsaers AJ, et al. Effects of the cyclooxygenase inhibitor, piroxicam, in combination with chemotherapy on tumor response, apoptosis, and angiogenesis in a canine model of human invasive urinary bladder cancer. Mol Cancer Ther 2003;2:183-188.
40. Knapp DW, Glickman NW, Widmer WR, et al. Cisplatin versus cisplatin combined with piroxicam in a canine model of human invasive urinary bladder cancer. Cancer Chemother Pharmacol 2000;46:221-226.
41. Schmidt BR, Glickman NW, DeNicola DB, et al. Evaluation of piroxicam for the treatment of oral squamous cell carcinoma in dogs. J Am Vet Med Assoc 2001;218:1783-1786.
42. Boria PA, Murry DJ, Bennett PF, et al. Evaluation of cisplatin combined with piroxicam for the treatment of oral malignant melanoma and oral squamous cell carcinoma in dogs. J Am Vet Med Assoc 2004;224:388-394.
43. Henry CJ, McCaw DL, Turnquist SE, et al. Clinical evaluation of mitoxantrone and piroxicam in a canine model of human invasive urinary bladder carcinoma. Clin Cancer Res 2003;9:906-911.
44. Knapp DW, Richardson RC, Chan TC, et al. Piroxicam therapy in 34 dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med 1994;8:273-278.
45. Robertson SA, Taylor PM. Pain management in cats—past, present and future. Part 2. Treatment of pain—clinical pharmacology. J Feline Med Surg 2004;6:321-333.
46. Heeb HL, Chun R, Koch DE, et al. Single dose pharmacokinetics of piroxicam in cats. J Vet Pharmacol Ther 2003;26:259-263.
47. Slingsby LS, Waterman-Pearson AE. Comparison between meloxicam and carprofen for postoperative analgesia after feline ovariohysterectomy. J Small Anim Pract 2002;43:286-289.
48. Wilson DV, Evans AT, Miller R. Effects of preanesthetic administration of morphine on gastroesophageal reflux and regurgitation during anesthesia in dogs. Am J Vet Res 2005;66:386-390.
49. Foss JF, Yuan CS, Roizen MF, et al. Prevention of apomorphine- or cisplatin-induced emesis in the dog by a combination of methylnaltrexone and morphine. Cancer Chemother Pharmacol 1998;42:287-291.
50. Hofmeister EH, Egger CM. Transdermal fentanyl patches in small animals. J Am Anim Hosp Assoc 2004;40:468-478.
51. Lascelles BD, Robertson SA. Use of thermal threshold response to evaluate the antinociceptive effects of butorphanol in cats. Am J Vet Res 2004;65:1085-1089.
52. Lascelles BD, Robertson SA. Antinociceptive effects of hydromorphone, butorphanol, or the combination in cats. J Vet Intern Med 2004;18:190-195.
53. Robertson SA, Taylor PM, Lascelles BD, et al. Changes in thermal threshold response in eight cats after administration of buprenorphine, butorphanol and morphine. Vet Rec 2003;153:462-465.
54. Grond S, Radbruch L, Meuser T, et al. High-dose tramadol in comparison to low-dose morphine for cancer pain relief. J Pain Symptom Manage 1999;18:174-179.
55. Leppert W, Luczak J. The role of tramadol in cancer pain treatment—a review. Support Care Cancer 2005;13:5-17.
56. Wu WN, McKown LA, Gauthier AD, et al. Metabolism of the analgesic drug, tramadol hydrochloride, in rat and dog. Xenobiotica 2001;31:423-441.
57. KuKanich B, Papich MG. Pharmacokinetics of tramadol and the metabolite O-desmethyltramadol in dogs. J Vet Pharmacol Ther 2004;27:239-246.
58. Mastrocinque S, Fantoni DT. A comparison of preoperative tramadol and morphine for the control of early postoperative pain in canine ovariohysterectomy. Vet Anaesth Analg 2003;30:220-228.
59. Parker R. Pharm profile: tramadol. Compend Cont Educ Pract Vet 2004;26:800-802.
60. Radulovic LL, Turck D, von Hodenberg A, et al. Disposition of gabapentin (neurontin) in mice, rats, dogs, and monkeys. Drug Metab Dispos 1995;23:441-448.
61. Chew DJ, Buffington CA, Kendall MS, et al. Amitriptyline treatment for severe recurrent idiopathic cystitis in cats. J Am Vet Med Assoc 1998;213:1282-1286.
62. Fan TM, de Lorimier LP, Charney SC, et al. Evaluation of intravenous pamidronate administration in 33 cancer-bearing dogs with primary or secondary bone involvement. J Vet Intern Med 2005;19:74-80.
63. Milner RJ, Farese J, Henry CJ, et al. Bisphosphonates and cancer. J Vet Intern Med 2004;18:597-604.
64. Alimi D, Rubino C, Pichard-Léandri E, et al. Analgesic effect of auricular acupuncture for cancer pain: a randomized, blinded, controlled trial. J Clin Oncol 2003;21:4120-4126.
65. Deng G, Cassileth BR, Yeung KS. Complementary therapies for cancer-related symptoms. J Support Oncol 2004;2:419-426.
Newsletter
From exam room tips to practice management insights, get trusted veterinary news delivered straight to your inbox—subscribe to dvm360.