Virginia R. Fajt, DVM, PhD
Articles by Virginia R. Fajt, DVM, PhD
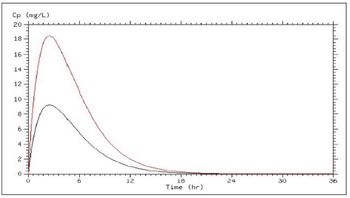
In most cases, we administer drugs at a different site than we want to drug to act. Understanding how drugs get to their site of action and how long they stay there is essential to making therapeutic decisions about which drug, what route, how much, how often, and for how long.

The Food and Drug Administration Center for Veterinary Medicine (FDA CVM) approves drug labels. The Environmental Protection Agency approves pesticides and products used on premises. State Boards of Pharmacy regulate the practice of pharmacy and drug dispensing. State Boards of Veterinary Medicine regulate the practice of medicine.

The science of how drugs work on the body (or the microorganism or parasite) is pharmacodymanics (its counterpart being pharmacokinetics, how the body works on the drug). In this section, the basic concepts of drug concentration and drug action are followed by a review of the mechanisms of action of the major drug groups used in food animal practice including NSAIDs, glucocorticoids, reproductive drugs, antimicrobials, and parasiticides.

At the time of this writing, the focus on farm animals by the media (and likely therefore consumer perception) seems to be on antimicrobial use in animal agriculture and on farm animal welfare.
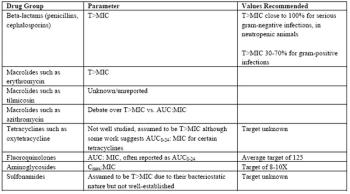
Traditionally, we have assumed that if a bacterial pathogen is "susceptible" to an antimicrobial, we just use the dose on the bottle or in a formulary, and the infection will be eliminated. The increasing incidence of "resistant" pathogens, i.e., pathogens requiring high concentrations of antimicrobials such that they become untreatable, has focused attention on identifying ways to reduce the selection for resistant organisms.

Drugs approved in the U.S. specifically for analgesia in cattle do not exist.

The design of antimicrobial regimens is addressed in the next section in these proceedings ("Antimicrobial Therapy: Regimen Design"), but the concepts within regimen design related to determining the concentration of drug required to inhibit growth of bacterial pathogens deserve a more thorough discussion. Antimicrobial susceptibility testing must not be viewed as a black box into which a veterinarian places a clinical sample of an infected site and receives a "yes" or "no" from the diagnostic laboratory.

One can usually find many sources of information about drugs: FDA website, drug company websites and technical reports, VIN, journals, trade magazines, and so on. The important skill required of veterinarians is to assess that information to determine its usefulness in your daily practice. Below are some principles of evaluating drug information, with the goal of improving treatment and the practice of medicine.
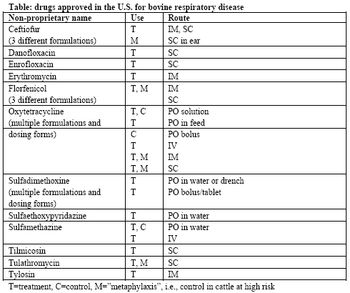
Bovine respiratory disease complex includes bacterial components, which cause the classic clinical signs of lethargy, depression, and fever, with variable nasal discharge, cough, or other signs. This bacterial component of BRD (most commonly Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis) may be treated with antimicrobial drugs designed to kill or inhibit the growth of the pathogenic bacteria.

The Food and Drug Administration Center for Veterinary Medicine (FDA CVM) approves drug labels. The Environmental Protection Agency approves pesticides and products used on premises.

At the time of this writing, the focus on farm animals by the media (and likely therefore consumer perception) seems to be on antimicrobial use in animal agriculture and on farm animal welfare.

The design of antimicrobial regimens is addressed in the next section in these proceedings, but the concepts within regimen design related to determining the concentration of drug required to inhibit growth of bacterial pathogens deserve a more thorough discussion.
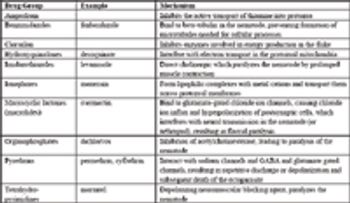
The science of how drugs work on the body (or the microorganism or parasite) is pharmacodymanics (its counterpart being pharmacokinetics, how the body works on the drug). In this section, the basic concepts of drug concentration and drug action are followed by a review of the mechanisms of action of the major drug groups used in food animal practice including NSAIDs, glucocorticoids, reproductive drugs, antimicrobials, and parasiticides.
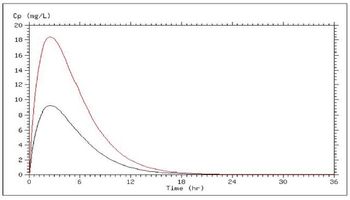
In most cases, we administer drugs at a different site than we want to drug to act. Understanding how drugs get to their site of action and how long they stay there is essential to making therapeutic decisions about which drug, what route, how much, how often, and for how long.

Recently, the use of antimicrobials in food animals has been scrutinized by the general public, by federal legislators, and by public health organizations. Some of these concerns relate to the use of antimicrobials as growth promotants, while some relate to the use of antimicrobials in food animals in general.

Drugs approved in the U.S. specifically for analgesia in cattle do not exist. There are guidance documents from the FDA Center for Veterinary Medicine for companies that would like to have an NSAID approved for pain relief

One can usually find many sources of information about drugs: FDA website, drug company websites and technical reports, VIN, journals, trade magazines, and so on. The important skill required of veterinarians is to assess that information to determine its usefulness in your daily practice.
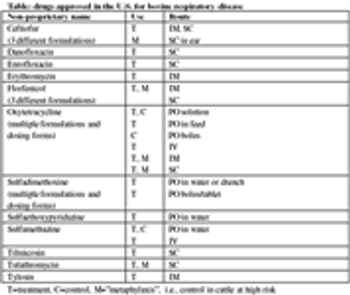
Bovine respiratory disease complex includes bacterial components, which cause the classic clinical signs of lethargy, depression, and fever, with variable nasal discharge, cough, or other signs. This bacterial component of BRD (most commonly Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis) may be treated with antimicrobial drugs designed to kill or inhibit the growth of the pathogenic bacteria.











