Michael D. Apley, DVM, PhD, DACVCP
Articles by Michael D. Apley, DVM, PhD, DACVCP

The capacity to experience pain is considered to have a protective role by eliciting behavioral responses aimed at reducing further tissue damage and enhance wound healing. However, persistent pain syndromes offer no biological advantage and are associated with suffering and distress.

Antimicrobial efficacy in cattle can be evaluated through clinical studies including a negative control group. To be included here, the study must have specified that the subjects were randomized, the evaluators were masked to treatment, and that statistical analysis was applied.

In this session we will take an evidence-based medicine approach to ancillary therapy of bovine respiratory disease, bovine toxic mastitis, bovine neonatal enteric disease, and retained placenta/metritis. The literature reviewed here is not presented as being all-inclusive, but rather as a summary of many commonly cited articles on these subjects.

The most critical bill related to the practice of veterinary medicine on the date these proceedings were prepared is HR 1549 - The Preservation of Antibiotics for Medical Treatment Act of 2009. The companion bill in the Senate is S 619

This presentation attempts to summarize some of the major concerns in resistance development along with key articles explaining relevance, epidemiology, and prevalence. It is not intended to be an exhaustive review of the literature and the interested practitioner should use the cited literature herein as a basis for continued, extended reading.
The short answer is "no" when we look for widespread, peer-reviewed evidence of untreatable infectious disease in food animals due to a microbial pathogen. However, there are some trends which bear watching. The evaluation of "untreatable disease" involves several inputs.

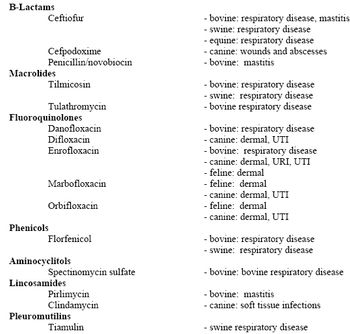
There are well thought out and clinically confirmed label regimens for label indications. For example, the antimicrobials labeled for individual animal treatment of respiratory disease in cattle and swine have label regimens which most likely give you the majority of the clinical results you will get.

"Susceptible" and "Resistant" are thrown around in the fields of microbiology, medicine, public health, and epidemiology with great frequency. Unfortunately, these classifications are often used in a manner inconsistent with their correct application.

This presentation attempts to summarize some of the major concerns in resistance development along with key articles explaining relevance, epidemiology, and prevalence. It is not intended to be an exhaustive review of the literature and the interested practitioner should use the cited literature herein as a basis for continued, extended reading.

The capacity to experience pain is considered to have a protective role by eliciting behavioral responses aimed at reducing further tissue damage and enhance wound healing. However, persistent pain syndromes offer no biological advantage and are associated with suffering and distress.
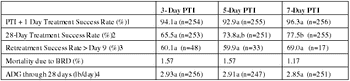
These proceedings present data related to the question of how long to wait after administering a single injection antimicrobial before applying success/failure criteria. More accurately, we will evaluate success/failure and mortality data based on administering a uniform regimen and then waiting different periods before applying success/failure criteria, and the animal subsequently being eligible for further therapy.

This checklist serves as a starting point for evaluating your applications of antimicrobials in food animals.

Antimicrobial efficacy in cattle can be evaluated through clinical studies including a negative control group. To be included here, the study must have specified that the subjects were randomized, the evaluators were masked to treatment, and that statistical analysis was applied. Much of the data were compiled from Food and Drug Administration Freedom of Information (FOI) summaries for veterinary drug approvals.

In this session we will take an evidence-based medicine approach to ancillary therapy of bovine respiratory disease. The literature reviewed here is not presented as being all-inclusive, but rather as a summary of many commonly cited articles on these subjects. The citations are primarily peer reviewed, but some are from freedom of information (FOI) summaries and a few are proceedings papers or abstracts.









