Articles by David A. Rhoda, DVM

Everything we have discussed to this point is actually about establishing a records plan that will allow us to serve the dairy industry of the future using the records that are efficiently gathered so we are not spending all of our time keeping or organizing the records. As our herds get larger, as the number of animals examined at any one time increases, as we are examining groups of diverse animals at one time, and as we struggle to maintain a cow side presence, the organization of the records becomes more critical.

The agricultural community is an extremely small percent of the general population and much of that population lives in densely populated areas of the country. They draw their perceptions of food animal care from their experiences and perceptions about zoos, their own companion animals, and the visual stories presented electronically from opponents of the animal industry.

Production medicine probably means something different to each of us, which has also been the case with written treatment plans definitions, our standards for accountability of welfare, defining the KPI's to monitor, and our drug protocols.
Opponents of food animal use rhetoric and disturbing images to incriminate lack of welfare, criticize drug usage, and incriminate modern care practices if they weren't the same method of care as in the past. They have an audience of consumers that have little or no knowledge of food animal care.

The request for a written treatment protocol is made frequently and easily by people that don't have to accomplish the task. This request is made with the best intentions and it is necessary that we accomplish this, but it is not quite as simple as it sounds.
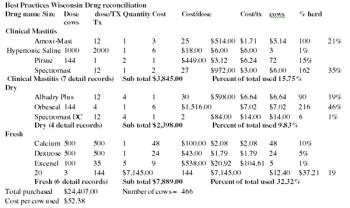
Supervision of the drug usage on the dairy is a foundation stone for achieving the VCPR and is referenced frequently in the regulatory documents that we are obligated to comply with. In 1996 the Animal Medical Drug Usage Clarification Act (AMDUCA) became the law that regulates extra-label drug usage (ELDU).
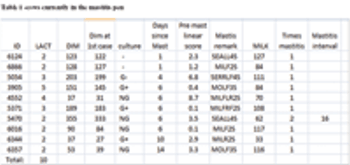
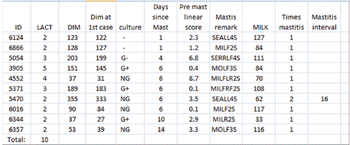
We have talked about supervision and finding treatment failures and conditions that have low odds of successful treatment in an efficient manner that is cost efficient for the dairy, good welfare for the cow and work we'd like to do. These are hollow words unless it can be delivered to the cows in need on a timely basis.

The regulatory agencies that license and regulate us include the requirement that we maintain a valid veterinary client patient relationship (VCPR).
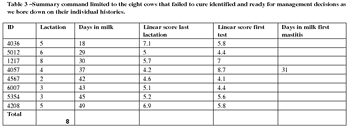
Anyone can and everyone does deliver key performance indicators (KPI's) to the dairy who is a stakeholder of the dairy either as an employee of the farm or as a business that is supplying materials or working for the dairy.









