Thoracic radiographic interpretation: The mediastinum (Proceedings)
Mediastinal abnormalities, including cardiac disease, are common causes of clinical signs related to the thorax. By definition, the mediastinum is the midline potential space formed between the two pleural cavities and includes the medial portions of the right and left parietal pleura (also called the mediastinal pleural) and the space formed between these serosal membranes.
Mediastinal abnormalities, including cardiac disease, are common causes of clinical signs related to the thorax. By definition, the mediastinum is the midline potential space formed between the two pleural cavities and includes the medial portions of the right and left parietal pleura (also called the mediastinal pleural) and the space formed between these serosal membranes. The mediastinum is incomplete and fenestrated in the dog and cat so that transudates and modified transudates are typically bilateral effusions, whereas exudates are unilateral effusions. The mediastinum can be divided into thirds in a cranial to caudal direction with each part then being divided into dorsal and ventral section. Table 1 documents the specific structures that are normally present in each of these spaces within the mediastinum.

Table 1: Normal structures present in the different parts and segments of the mediastinum. The (*) indicates those structures that are normally NOT seen on thoracic radiographs.
A systematic approach to evaluating the mediastinum is critical to establishing normality for a given small animal. One can think of roentgen abnormalities of the mediastinum as either primary (an abnormality of the mediastinum itself) or secondary (an abnormality caused by a mediastinal structure or organ). Examples of primary mediastinal abnormalities would include abnormal fluid or gas collections within the mediastinum. Examples of secondary mediastinal abnormalities would include lymphomegaly, cardiac abnormalities, esophageal disorders, tracheal disorders and abnormal hemorrhage of tumors of the thymus.
Objectives of the Presentation
1. Provide practitioners with a basic interpretation paradigm for the evaluation of the mediastinal structures of the small animal thorax.
2. Provide a summary schema for evaluating mediastinal abnormalities.
3. Think in terms of next step and how to get a cytologic diagnosis in as non-invasive a fashion as possible.
Key Etiologic and Pathophysiologic Points
1. Technical factors including technique, phase of respiration and the positioning of the patient have to be taken into account when interpreting thoracic radiographs. High quality, well positioned thoracic radiographs are the most critical first step to evaluating patients with intrathoracic disease (and possibly mediastinal abnormalities).
2. The basic pathophysiology related to diseases of the mediastinal structures should be understood for completing the exercise in the formulation of differentials for the described mediastinal abnormalities.
3. The description of the mediastinal abnormalities is NOT the end point but should be considered the initiation point for formulating a reasonable list of differential diagnoses for the described roentgen signs.
Key Clinical Diagnostic Points
1. One should try to compartmentalize radiographic abnormalities into extrathoracic, pleural, pulmonary and mediastinal (including cardiac), recognizing that any disease can be multicompartmental in nature.
2. One should try to determine the anatomic location of pathology within the lung first and foremost and then worry about the pulmonary pattern. Even though there may be several pulmonary patterns, one must identify the dominant pattern in order to evaluate for differentials.
3. Echocardiography can not diagnosis when a patient is in left sided heart failure with pulmonary edema.
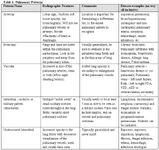
Table 1: Pulmonary Patterns
Key Therapeutic Points
1. Only start to treat for a specific disease once that disease has been confirmed and is based on a solid physical examination and diagnostic radiographs.
2. DO NOT treat a dog for pulmonary edema if you have only taken a right lateral projection that is on expiration as this most likely is artifact and not truly edema.
Key Prognostic Points
1. The anatomic localization of the disease process to the mediastinum and the subsequent differentials will then determine the prognosis.
Discussion: The Mediastinal Space
The mediastinum is formed by the reflection of the two parietal pleural mesothelial linings. Some questions that are pertinent to the mediastinal space, aside from the cardiac silhouette would include: Is the mediastinum normal for size, margins, shape and opacity? Is there a mediastinal mass or any fluid? Is there mediastinal air and if yes, is it focal and contained in a mediastinal structure or is it generalized within the mediastinal space? Is there a mediastinal shift? If the answer to any of these questions is yes, then the practitioner should define the exact anatomic position of the abnormality. In other words is the abnormality focal (cranial, middle caudal and dorsal or ventral in position) or generalized.
The Cranial Mediastinum
The dorsal aspect of the cranial mediastinum is characterized by soft tissue opacity that is divided by a radiolucent tube called the trachea. Other anatomic structures that are present in the dorsal aspect of the cranial mediastinum include the cranial thoracic vessels (aortic arch, brachiocephalic trunk, left subclavian, azygous vein, cranial vena cava), fat, nerves (vagosympathetic trunk), esophagus and cranial mediastinal lymph nodes. The trachea and its walls are normally the only structure that is visualized on the lateral and VD/DV radiograph. The trachea is normally located in a central to right sided position on the VD/DV radiograph. The trachea normally diverges away from the vertebral column on the lateral radiograph.
The ventral compartment of the cranial mediastinum is made up the mediastinal reflection of the right and left cranial lung lobes. On the VD radiograph the ventral mediastinal reflection can be seen and defined by the left and right cranial lung lobes. The left cranial lingula will extend into the right cranial hemithorax in large breed dogs, but not cats, brachiocephalic dogs or obese small dogs.
The thymus is a normal structure seen in immature cats and dogs that has a typical appearance of a triangular soft tissue opacity extending from a craniomedial position to a caudolateral position in the cranioventral mediastinal reflection. This called the "sail" sign. On the lateral radiograph a curvilinear soft tissue structure may be seen (inconsistent even if the thymus is present on the VD/DV radiograph). While the thymus is located on the left of the vertebral column, enlargement of the sternal lymph node will result in widening of the ventral mediastinal reflection in a fusiform to oval shape on a rightward or midline position superimposed over the cranial thoracic vertebral bodies on the VD/DV radiograph. Also within the cranial ventral mediastinal reflection, the internal thoracic vessels (artery and vein) are found. On the VD radiograph, the cranial vena cava and trachea will be located just rightward of the thoracic vertebral bodies.
Is there air present in the mediastinum? Is the air focal or generalized? Generalized gas accumulation within the cranial or caudal mediastinum must be distinguished from air within the esophagus. Cervical trauma, external penetrating wounds, esophageal or tracheal rupture/tear, venipuncture or transtracheal washes can result in a pneumomediastinum. As air accumulates within the cranial mediastinum, various structures and edges of soft tissues that normally will border efface and become apparent. If the pneumomediastinum becomes severe then the air will dissect caudally into the retroperitoneal space via the aortic hiatus through the diaphragm. A pneumomediastinum will only be seen on the lateral radiograph unless the VD/DV is obliqued. A pneumomediastinum may lead to a pneumothorax but a pneumomediastinum will not result from a pneumothorax as the air collapses the mediastinal space between the two pleural reflections. The key to diagnosing a pneumomediastinum is the ability to see soft tissue structures within the cranial mediastinum that are usually not seen on normal radiographs. These structures would include the outer (normally not seen) and the inner (normally seen) margins of the tracheal wall, the entire thoracic aorta, the great vessels of the cranial mediastinum, the azygous vein, and the longus colli muscles. Gas on the outside of the trachea, is called the tracheal stripe sign and is consistent with a pneumomediastinum. Gas within the cranial thoracic esophagus will cause the ventral border of the esophagus to border efface with the dorsal border of the trachea creating what is called the tracheo-esophageal stripe sign.
Is there a mediastinal mass or fluid? Common causes of mediastinal masses include lymph node mass(es) or enlargement, thymic mass (thymoma or thymic lymphoma), esophageal enlargement (dilated as with generalized or partial megaesophagus), mediastinal hemorrhage (thymic rupture in an immature dog or hemorrhage secondary to trauma or coagulapathies). Typically, on radiographs of animals with craniodorsal mediastinal masses, there will be symmetrical widening of the cranial mediastinum on the ventrodorsal radiograph with caudal displacement of the right and left cranial lung lobes. Mediastinal tumors usually arise from the thymus, lymph nodes, ectopic thyroid tissue or connective tissues of the dorsal and cranial mediastinal structures. Mediastinal fluid may accumulate due to the presence of a mass, hemorrhage or mediastinitis, or can be secondary to esophageal perforation.
Mediastinal masses can also be accompanied by pleural fluid. The presence of the fluid can make diagnosis of the mediastinal masses difficult. Dorsal deviation of the trachea in the presence of a moderate or severe volume of pleural fluid cannot be used to infer the presence of a mediastinal mass, even with caudal displacement of the tracheal carina. Repeating radiographs following thoracocentesis can be helpful. Positional films may be more useful for evaluating the cranial mediastinum. An erect horizontal beam DV or VD film is preferred. Using this positional radiograph, the pleural fluid will drain to the caudal thorax (now in a down, gravity position) and the lungs to outline the lateral margins of the cranial mediastinum quite clearly. An alternative technique is to perform a non-selective angiogram cranial vena cava. A large bore catheter is placed in either jugular vein and a bolus of water-soluble iodinated contrast medium (400 to 800 mg I/kg) is injected as quickly as possible. Mid way or simultaneous to the end of the contrast medium injection, a lateral radiograph is obtained centered over the thorax. If a cranial mediastinal mass is present there will be dorsal displacement, compression and/or distortion of the cranial vena cava. The cranial vena cava should also be evaluated for the presence of intraluminal abnormalities. Filling defects within the contrast that have a consistent appearance on multiple films suggest thrombus formation or vascular invasion by tumor. A crude assessment of cardiac size may also be made by this technique. If a pericardial abnormality is suspected (pericardial effusion or peritoneopericardial diaphragmatic hernia), the true heart versus the cardiac silhouette can be determined.
Radiographic signs of a mass or fluid can include increased overall soft tissue opacity on the lateral film, elevation of the trachea, widening of the cranial mediastinum to exceeds the width of the spine on the VD or DV film (except in brachycephalic breeds or obese patients), blurring or obliteration of the cranial border of the heart.
Is there an ipsilateral or contralateral mediastinal shift? A mediastinal shift is secondary to a pleural or pulmonary abnormality. It may occur either due to volume loss or increased volume in the adjacent lung lobe or the pleural space in one hemithorax. An ipsilateral mediastinal shift is secondary to volume loss (atelectasis) of a cranial lung lobe or a pleural or lobar mass resulting in a contralateral mediastinal shift. The lungs should be evaluated for evidence of a mass, overinflation or volume loss and the pleural space for a mass and/or fluid. A mediastinal shift will occur within minutes after the animal is anesthetized. A common cause of atelectasis can be secondary to prolonged lateral recumbency, general anesthesia or following thoracic surgery.
The sternal lymph node is located in the ventral compartment of the cranial mediastinum. It is located dorsal to the second or third sternebrae on the lateral radiograph. The sternal lymph node receives lymphatic drainage from the pleural and peritoneal surfaces of the diaphragm and organs of the abdomen. Sternal lymphadenopathy is commonly associated with multicentric lymphoma in dogs or peritoneal inflammatory or neoplastic disorders. The sternal lymph node is easier to recognize in the dog than the cat because of the ventral lung lobe mediastinal reflections. These mediastinal reflections are not as prevalent in the cat and thereby the sternal lymph node is seen as an ill-defined oval increase in soft tissue opacity dorsal to the second sternebrae. On the VD radiograph of a dog with sternal lymphadenopathy, there will be an "S" deformity and widening to the ventral cranial mediastinal reflection with focal retraction of the cranial lung lobes (not symmetrical widening as with a dorsal cranial mediastinal mass).
The esophagus
Is the esophagus normal? The normal esophagus is not visualized. The esophagus runs from a dorsal cranial mediastinal position to a caudodorsal mediastinal position over the heart base area. The esophagus inserts into a slightly leftward opening in the mid dorsal diaphragm, at the esophageal hiatus. The esophagus lies just to the left and dorsal aspect of the trachea. In the caudal mediastinum the esophagus is in a central position superimposed over the caudal thoracic vertebrae. In the cranial thorax and just cranial to the thoracic inlet, the esophagus lies in a dorsolateral position (leftward) and can cause the trachealis muscle and dorsal tracheal membrane to indent in to the tracheal lumen. This is called a redundant dorsal tracheal membrane and has been considered a type I tracheal collapse. However, this can be seen in numerous dogs without clinical signs of coughing and should not be considered a significant abnormality without the appropriate clinical context.
Many normal dogs will have a short, thin, linear gas shadow within the esophagus just cranial to the tracheal bifurcation, most commonly identified on the left lateral radiograph. This should only be regarded as abnormal if it persists unchanged on multiple films. Heavy sedation and anesthesia frequently cause esophageal dilation so megaesophagus can only be diagnosed in conscious animals. Moderate to severe dilation of the cranial thoracic esophagus will cause a mass effect and displace the trachea in a ventral and rightward position. The apposed esophageal and tracheal walls are seen as a soft tissue stripe if the esophagus is gas filled and may be confused with a pneumomediastinum. But this stripe is thicker than the tracheal stripe. The walls of the caudal esophagus are outlined by luminal gas as two thin soft tissue stripes converging on the esophageal hiatus of the diaphragm. A fluid filled dilated esophagus does not usually have radiographically discrete margins and appears as an increased opacity in the caudodorsal thorax on the lateral film. A VD or DV view confirms the increased opacity is on midline and prevents confusion with pulmonary pathology. Sharpei breeds will have a redundant esophagus at the thoracic inlet that can be seen without clinical signs and thereby the clinical significance of this finding is not known.
Is the esophagus dilated? It is important to distinguish segmental from generalized megaesophagus. Dilation of the esophagus cranial to the heart base in immature animals is consistent with a vascular ring anomaly or a cranial/middle mediastinal esophageal stricture. Acquired segmental megaesophagus is rare but may occur as the result of esophageal stricture formation or secondary to a focal partial obstruction (foreign body or mass). Generalized megaesophagus is diagnosed if the entire esophagus is dilated. It may be congenital or acquired. The list of causes of acquired megaesophagus is too numerous to mention here and the reader is referred to textbooks of small animal medicine for the appropriate work up of dysphagia and regurgitation. Several common causes of acquired generalized megaesophagus may be due to myasthenia gravis, neuromuscular disorders, and primary esophageal disorders (esophagitis, diverticulum or partial/complete obstruction), acetylcholinesterase inhibitors (organophosphate toxicity) or endocrinopathies but is commonly idiopathic. Megaesophagus is often accompanied by aspiration pneumonia that is sometimes best-evaluated using right and left lateral radiographs. Careful inspection of the lung parenchyma over the cardiac silhouette is required to visualize the presence of parenchymal abnormalities. However, animals with severe pulmonary disease and dyspnea may exhibit esophageal dilation due to aerophagia, so the presence of esophageal disease can only be accurately assessed when the dyspnea has resolved.
Is there an esophageal foreign body? Is the esophagus perforated? Esophageal foreign bodies are more commonly seen in dogs and only rarely in cats. The common sites for objects to become trapped are the thoracic inlet, heart base and esophageal hiatus. In cats foreign bodies may also become trapped at the cricopharyngeal sphincter. Bones are easy to identify but soft tissue opaque objects (cloth material, rubber material) are often overlooked. This is especially true as owners can confuse vomiting with regurgitation thereby directing the clinician's attention to the abdomen. The esophagus is usually dilated cranial to the lesions and gas may outline the foreign object. The mediastinum should be carefully evaluated for evidence of an esophageal perforation. Perforation results in a mediastinitis that is seen as a peri-esophageal accumulation of fluid and mediastinal gas. This may progress to pleural effusion and a pneumothorax.
Is there an esophageal mass? An esophageal mass effect can be seen with foreign bodies, parasitic granulomas or primary esophageal tumors. Differentiation may not be able to be done without an appropriate contrast medium study (esophagram) using a barium sulfate paste, liquid and barium sulfate mixed with food. Esophageal tumors are rare, but can occur, (Figure 69). Squamous cell carcinomas and leiomyomas are the more tumors of the esophagus. A tumor can be present at the gastroesophageal junction and may not be seen unless fluoroscopy is used to dynamically evaluate this area. Parasitic granulomas secondary to Spirocerca lupi will be seen in the mid thoracic esophagus. These parasites are rare in the United States. Any esophageal mass can cause hypertrophic osteopathy with resulting palisading periosteal reactions along the diaphyses of long bones. The original presentation in these dogs is because of the limb swelling and lameness.
Trachea
Is the trachea normal? The caudal cervical trachea is usually included on a lateral thoracic radiograph. The trachea can be seen as the radiolucent (gas filled) tube extending from the caudal cervical region through the thoracic inlet and in a dorsal 2/3 position within the cranial thorax. The trachea terminates at the carina, which is the bifurcation of the caudal thoracic trachea into the caudal mainstem bronchi. The normal trachea at the thoracic inlet is between 15 and 20% of the thoracic inlet internal dimension as measured on the lateral radiograph. For bulldogs and other brachycephalic breeds this measurement can approach 12% and still be considered normal.
Is the size of the trachea narrow (small luminal diameter)? If the trachea is narrowed is the lesion focal, generalized, fixed or dynamic? A hypoplastic trachea will be seen throughout the length of the cervical and thoracic trachea. A hypoplastic trachea exists if the measured luminal diameter is less than 12% of the thoracic inlet internal measured dimension. Typically dogs with hypoplastic tracheas will present early in life and will have other components of a brachycephalic syndrome.
If a clinical suspicion of tracheal disease exists radiographs of the entire trachea should be obtained during inspiration and expiration. Tracheal collapse is a common clinical entity in small and toy breed dogs). Incompletely formed tracheal rings and a flaccid or redundant tracheal membrane cause narrowing of the lumen. This is usually a dynamic lesion that is it changes with the phase of respiration. On inspiration there is negative pressure within the cervical portion of the trachea and it will collapse or narrow. On expiration, positive pressure within the thorax causes narrowing or collapse of the intrathoracic trachea and in some cases the main stem bronchi. Uniform narrowing of the tracheal lumen is seen in tracheal hypoplasia, especially in brachycephalic breeds. Apparent uniform narrowing of the trachea may be seen in dogs due to hemorrhage caused by intoxication by vitamin K antagonist rodenticides. In these cases, the air shadow of the lumen will be much smaller than the outline of the tracheal rings. A focal fixed narrowing of the trachea is most likely a post-traumatic stricture. These are usually located at or just caudal to the thoracic inlet. These lesions are easily overlooked as they are small and may be partly obscured by the overlying shoulder musculature. The stenosis is the result of a traumatic tear of the trachea or overenthusiastic inflation of the cuff of an endotracheal tube.
Is there a mass or nodules within the trachea? Is there a foreign body present? Occasionally tumors arise from the tracheal mucosa and result in mass or nodule formation. Multiple nodules located at the tracheal bifurcation are caused by infestation with the filarial nematode, Osleris osleri. Tracheal foreign bodies are rare. Mineral opacity or metallic objects are easy to detect but fragment of wood or leaves are more common and being soft tissue opacity are more difficult to detect.
Is the trachea displaced (mass effect resulting in tracheal displacement)?
Displacement of the tracheal is a very useful radiographic sign. Dorsal displacement of the cranial thoracic trachea occurs as a result of a cranial mediastinal mass. Keep in mind this appearance can be seen in some dogs, especially if the head is tucked down when the radiograph is taken. Before concluding a mass is present the cranial mediastinum should be evaluated on both radiographic views. Also repeating the lateral radiograph with the dog's neck in an extended position will confirm that the apparent dorsal deviation was secondary to neck flexion (positional) and not a true mediastinal mass. In young dogs with a persistent right aortic arch or other vascular ring anomalies will result in ventral deviation of the trachea on the lateral radiograph. On the VD radiograph the trachea will be displaced toward the left side and can be seen to the left of the vertebrae at the thoracic inlet. A heart bass mass may cause dorsal and rightward displacement of the distal trachea while generalized cardiomegaly causes dorsal displacement of the trachea.
Ventral displacement of the trachea at the level of the carina on the lateral radiograph is usually secondary to either tracheobronchial lymph node enlargement or a dorsal and central pulmonary or mediastinal massThere are three primary lymph centers associated with the tracheobronchial lymph nodes. Depending on the abnormality, there is one or more of these lymph nodes that will enlarge. The right tracheobronchial lymph node is located between the right cranial lobe bronchus and the trachea. Enlargement of this lymph node will cause lateral and ventral displacement of the right cranial lobar bronchus. The central tracheobronchial lymph node is located between the right and left caudal mainstem bronchi just caudal to the carina. There will be ventral displacement of the carina and caudal mainstem airways as well as widening of the caudal mainstem bronchi when the central tracheobronchial lymph node is enlarged. The left tracheobronchial lymph node is located between the cranial sub-segmental bronchus of the left cranial lung lobe and the trachea. Enlargement of this bronchus will cause ventral and lateral displacement of the associated left cranial lung lobe bronchus. Tracheal displacement is not a reliable radiographic sign if a moderate or large volume of pleural fluid is present. The trachea can appear to be dorsally and caudally displaced in this situation and a cranial mediastinal mass can neither be denied nor confirmed. As previously mentioned, erect, horizontal beam radiographs after thoracocentesis may help define the presence of any cranial mediastinal abnormalities.
Pulmonary masses can displace the trachea and mainstem airways in any direction depending on the anatomic origin and size of the pulmonary mass. Evaluation of the bronchi for possible mass invasion should be done, as bronchoscopy may then be beneficial for obtaining a diagnosis.
Major bronchi
Are the bronchi normal? The major bronchi have walls that are thick enough to be radiographically visible and should not be mistaken for an abnormal bronchial pattern. Each bronchus should be carefully evaluated for evidence of narrowing, enlargement, foreign bodies or displacement. One has to be familiar with the normal bronchial anatomy and origin of each bronchus from the trachea for accurate interpretation. Straight lateral radiographs are required for best evaluation of the lobar bronchi. Lobar bronchi can be typically seen to the level of the second and third generation. Linear mineralizations can be seen within the walls of the trachea and mainstem airways in older geriatric dogs and considered normal. Dystrophic mineralization of the tracheal rings is also an inconsistent finding that is considered normal. This change can also be seen in dogs that have pituitary dependent hyperadrenocorticism. As the bronchi move away from the central trachea, the airways will narrow or converge into the periphery. At times the only indication of where the bronchus is located may be because of the location of the pulmonary artery and vein.
Are the bronchi narrowed? Collapse of the mainstem bronchi during expiration is common in dogs with tracheal collapse. This is an important finding as bronchial collapse is not amenable to surgical correction. In some cases collapse of the bronchi can only be demonstrated by fluoroscopy.
Are the bronchi enlarged? Bronchiectasis is defined as the chronic, abnormal dilation of the bronchi or bronchioles as a sequela of inflammatory lung disease of chronic obstructions associated with pneumonia and heavy mucous secretions. These changes are commonly associated with bronchopneumonia or recurrent lung infections as the ciliary apparatus of the bronchi and trachea is normally abnormal as well. A dilated bronchus can be seen into a lobe that has acute pneumonia, but returns to normal after treatment. In true bronchiectasis, the diameter of the bronchi will not return to normal and in fact may get larger over time. The different types of bronchiectasis include saccular, cylindrical (tubular) or a mixture of both. These are just anatomic descriptions as to what the abnormally dilated airways look like on radiographs and have nothing to do with the underlying pathophysiology of the disease process. At times the saccular form may appear like a cavitated lung lesion and horizontal beam, sternal recumbency radiographs may be necessary in order to differentiate between these two different radiographic lesions. In young dogs with recurrent infections, hereditary disorders of the ciliary apparatus (ciliary dyskinesis) should be evaluated for using electron microscopy or nuclear medicine studies that are specific for ciliary clearance.
Are the bronchi displaced? Displacement of the mainstem bronchi indicates enlargement of adjacent structures. Left atrial enlargement causes dorsal displacement of the left caudal lobar bronchus and rather than both bronchi being superimposed the bronchi are split on the lateral film. Severe enlargement will cause dorsal displacement of the right caudal lobar bronchus too. On the VD or DV film the caudal lobar bronchi are displaced laterally, resulting in a stirrup like appearance also described as the bowlegged cowboy. Enlargement of the left atrium is most often caused by mitral endocardiosis or dilated cardiomyopathy (DCM) in dogs and hypertrophic cardiomyopathy (HCM) in cats. Similar lateral displacement of the bronchi is seen with tracheobronchial lymph node enlargement. However on the lateral film, enlargement of these lymph nodes causes ventral displacement of the mainstem bronchi. Common causes of tracheobronchial lymph node enlargement include lymphoma, metastasis from primary pulmonary neoplasia, malignant histiocytosis or systemic mycosis.
The Middle Mediastinum.
The Cardiac Silhouette
Depending upon the cardiac abnormality radiology can be both highly sensitive and specific in diagnosing specific cardiac disorders. However, radiology can also be extremely insensitive and non-specific for other cardiac disorders. This may seem contradictory but keep in mind that we cannot see internal cardiac chambers, vessels, leaflets, etc. We can, however, extrapolate specific changes in the cardiac chambers from relatively non-specific changes in the overall size and shape of the heart. Radiology is most limited in the diagnosis of congenital heart disease and more useful for evaluating acquired cardiac disease. Although a disease specific diagnosis may not be made, valuable information about the severity of cardiac changes, degree of heart failure and response to therapy can be obtained from radiographs. Serial radiographic examinations over time can also document progression of cardiac changes, chamber enlargement and heart failure.
Radiographic Assessment of Cardiac Disease
There are a series of 5 questions that one should ask oneself when evaluating the cardiac silhouette for abnormality. The answers to these five questions should make sense based on the final diagnosis one makes. It may not be possible to arrive at a specific diagnosis, but render list of potential differentials that could fit the radiographic abnormalities that have been described. The five questions include: Is there any roentgen sign abnormalities associated with the cardiac silhouette? Is there evidence of cardiac enlargement? If so, can it be characterized as right sided, left sided or generalized. Is there any radiographic evidence of heart failure (specifically pulmonary edema or left heart failure and pleural effusion or right heart failure in the dog)? Is there any abnormality of the heart base area with abnormal enlargement of the descending aorta, aortic arch or main pulmonary artery segment on the VD/DV radiograph (must be positioned straight!!)? Is there any radiographic evidence of pulmonary over or under circulation or are there asymmetric changes associated with lobar pulmonary arteries and veins.
Each of these questions will be broken down and further reviewed in the following discussion. Remember that because of the tremendous breed variation in the dog, it is difficult to arrive at a basic formula for say confidently on every radiograph that the cardiac silhouette is normal or abnormal or even enlarged.
Based on the position of the heart, where is the apex located on the lateral and the VD/DV radiograph? The apex is the best external cardiac landmark that documents the division between the left and right sides of the cardiac silhouette. Remember though that the external structure that one is looking at is really the outer border of the pericardial lining and not the epicardial surface of the heart. Locating the apex is necessary to determine if the left or right side of the heart is enlarged. On the VD radiograph, the apex of the heart is can be determined by using the caudoventral mediastinal reflection. This reflection is located between the apex of the cardiac silhouette and the caudal left ventral aspect of the diaphragm. The apex of the cardiac silhouette is seen along the caudoventral aspect of the heart on the right lateral radiograph. On the left lateral radiograph, the cardiac silhouette shifts dorsal and lateral and the cardiac apex cannot be identified. Severe left-sided cardiomegaly may cause rightward displacement of the apex on VD or DV films and mimic the appearance of right-sided cardiomegaly. On right lateral radiograph, severe right-sided cardiomegaly will lift the apex off the sternum. On VD films leftward deviation of the apex may occur with right-sided cardiomegaly.
Is the overall cardiac size normal, reduced or enlarged? The size of a normal cardiac silhouette will vary considerably with breed and conformation in dogs and with age in cats. Additionally, since one is evaluated a disease process, the size of the cardiac silhouette will be dependent upon the specific etiology and the previous course of the disease. For example, a heart that is 3.5 intercostal spaces wide on a lateral radiograph would be quite normal in a Golden Retriever but enlarged in a Doberman pinscher. A reduced cardiac size is usually the result of a depleted circulating volume. Dehydration, hemorrhage and Addison's disease are common causes of this appearance. Microcardia may or may not be accompanied by a hyperlucent appearance to the lung and small pulmonary vessels.
Are any specific chambers or great vessels enlarged? Specific cardiac chambers and great vessels cannot be identified as such on radiographs but form specific portion of the cardiac outline. The outline of the heart is treated as a clock face and bulges or bumps described based on this clock analogy.
Left atrial enlargement in dogs is seen as a triangular bulge at the caudodorsal aspect of the heart on the lateral film. In cats the enlarged left atrium appears as a rounded bulge in the same location, altering the normal lemon like shape of the heart to kidney bean shaped. Left atrial enlargement causes elevation and sometimes compression of the left caudal stem bronchus and if severe the right also. On the VD, the enlarged left atrium spreads the mainstem bronchi, the "bowlegged cowboy" sign, and may result in a double edge at the caudal border of the heart silhouette (6 o'clock). In severe enlargement, the left atrial appendage is seen as a bulge on the left lateral border of the heart (3 o'clock) on the VD/DV radiograph.
Left ventricular enlargement results in straightening on the caudal cardiac border on the lateral film and an elongated heart on the VD. On the VD, the left heart border bulges and is closer to the thoracic wall than normal. The cardiac apex may be shifted to the right creating the impression of right cardiomegaly, so before deciding if an enlargement is left or right sided, always located the apex. Left ventricular hypertrophy is usually concentric (hypertrophic cardiomyopathy in cats, sub-aortic stenosis in dogs) and may produce minimal radiographic change in the size and shape of the heart unless the left atrium is enlarged or myocardial failure has occurred resulting in ventricular dilation.
Right atrial enlargement is rare as a discrete change with the exception of hypertrophic cardiomyopathy in the cat and tricuspid dysplasia in dogs or cats. It is seen as expansion and rounding of the cranial cardiac border just ventral to the trachea on the lateral film and a bulge on the right cranial heart border on the VD (9 - 11 o'clock).
Right ventricular enlargement results in rounding and cranial expansion of the cranial cardiac border on the lateral film and rounding of the right cardiac border which is closer to the thoracic wall than normal on the VD film . In some cases this produces the reverse D appearance on VD or DV radiographs.
Aortic enlargement is seen a bulge at the cranial aspect of the heart which blends with the cranial mediastinum on the lateral and a rounded "cap" on the cranial border of the heart on midline of the VD.
An enlarged main pulmonary artery may be seen as an increased opacity just cranial to the tracheal bifurcation on the lateral film when enlarged and a bulge at the left cranial border of the heart on the VD.
The size of the caudal vena cava varies considerably with the phases of the cardiac and respiratory cycles but it has been shown not to exceed the diameter of the descending aorta within the dog or cat being evaluated.
Are the pulmonary vessels small, normal or enlarged? Normal pulmonary arteries and veins should be approximately the same size. The cranial lobar vessels are assessed on the lateral film, where the artery is the more cranial and dorsal of the pair. The caudal lobar vessels are assessed on a VD or preferably DV film where the artery is the lateral vessel and the vein lies medially. An objective assessment of size is made by comparing vessels the cranial lobar vessels to the 4th rib and the caudal lobar vessels to the 9th rib where they cross it. Normal vessels should be equal to or smaller than the width of the proximal third of the corresponding rib. In some cases, especially congenital shunt lesions, there may be an increase in number of radiographically visible pulmonary vessels rather than enlargement of the hilar and mid zone portions of the vessels. In such cases, examination of the periphery and mid zones reveals an increased number of small vascular markings or a moderate to severe unstructured or reticular (honeycomb like) interstitial pattern. This is true for left to right shunting lesions such as a patent ductus arteriosus or a ventricular septal defect.
Is there evidence of Left Heart Failure (pulmonary edema in dogs)? Pulmonary venous enlargement is an early indicator of elevated left ventricular and left atrial diastolic pressures. As the left atrial pressure increases, the amount of fluid retention within the interstitial space will increase. This will result increased pulmonary opacity. As the interstitial edema progresses there will be flooding of the alveoli and an alveolar lung pattern can be seen. In dogs with chronic endocardiosis that acutely decompensate, a right caudal lung lobe distribution may be apparent. In our experience (although this has not been scientifically proven) dogs on chronic cardiac medications (enalapril and lasix) will have normal or small sized pulmonary veins, even when there is radiographic evidence of pulmonary edema.
Is there pleural fluid, hepatosplenomegaly or ascites? Right-sided congestive heart failure typically will result in caudal vena caval enlargement, hepatosplenomegaly and ascites prior to pleural effusion in the dog. In the cat, congestive heart failure can result in strictly pulmonary edema, pleural effusion or a combination of both. Usually ascites is a late feature of chronic cardiac decompensation in the cat.
Remember that the diagnosis of right-sided or left-sided congestive heart failure is a syndrome and is not a specific clinical diagnosis. The presence of right or left sided congestive heart failure is used to formulate a differential diagnosis for the other radiographic abnormalities that are present.
Differential diagnosis of cardiac disease
Formulating a differential diagnosis is simplified by considering heart disease as either acquired or congenital. Generally animals with signs of cardiac disease and aged under 5 years are more likely to have a congenital lesion. However, acquired diseases such as dilated and hypertrophic cardiomyopathy and heartworm disease occur in young animals.
Congenital cardiac disease may be classified into four major categories, which include: valvular stenotic lesions; valvular incompetence (atrioventricular dysplasias) lesions; shunting lesions; and complex congenital lesions that include combinations of these basic defects. A review of cardiac embryology is beyond the scope of these notes, but a basic understanding is needed in order to try to understand and evaluate dogs or cats with congenital heart defects. Echocardiography (including Doppler and Color flow imaging) and possibly angiocardiography may be required in order to fully define the extent and severity of the congenital defect. If right to left intracardiac lesions are suspected, the use of contrast (saline bubble) echocardiography can be useful. In small dogs and in cats, non-selective angiography is useful for the evaluation of right-sided lesions (atrioventricular dysplasia or pulmonic stenosis or complex lesions such as a tetralogy of Fallot).
Stenotic lesions most commonly affect the aortic or pulmonic valves. The outflow tract may be narrowed below the valve (sub-aortic stenosis most common), at the valve (valvular pulmonic stenosis most common), or distal to the valve (supravalvular lesion). The narrowing causes increased resistance to ventricular output resulting in concentric myocardial hypertrophy. Concentric hypertrophy may cause little alteration of the size and shape of the left ventricle. The right ventricle tends to become rounded, causing a rounded bulging right cardiac border on both lateral and VD films. The high-speed jet of blood pushed through the stenosis results in post stenotic dilation secondary of the artery just distal to the lesion to turbulence. Enlargement of the main pulmonary artery is best seen on the VD or DV film at the left cranial aspect of the cardiac silhouette (1-2 o'clock). Moderate or severe pulmonic stenosis can cause a reduction in size of the pulmonary vessels. Enlargement of the aortic arch and ascending aorta can be seen as a bulge on the cranial aspect of the heart on the lateral VD films (12 o'clock). If severe, this dilation will create the impression of an elongated cardiac shadow on the VD or DV film.
Valvular incompetence occurs due to dysplasia of the atrioventricular valves. This is a common anomaly in cats and may affect either or both the mitral or tricuspid valves. Moderate to severe atrial enlargement develops due to regurgitation of blood from the ventricle during systole. Myocardial failure ensues and severe generalized cardiomegaly is seen radiographically. These lesions are usually severe and significant cardiomegaly and failure occurs in quite young animals.
Intra or extra cardiac left-to-right hunting lesions are the result of a persistence of a portion of the fetal circulation (patent ductus arteriosus, PDA) or maldevelopment of cardiac structures (atrial and ventricular septal defects). Flow through the shunt is from left to right unless severe pulmonary hypertension exists. The radiographic changes in the heart are variable and nonspecific. Classically, a left to right PDA will result in enlargement of the descending aortic arch (ductus diverticulum), the main pulmonary artery and the left atrial appendage. If all three are present the three bulges results in the "three knuckle sign" on the VD or DV radiograph but more commonly only one or both of the great arteries is enlarged. Septal defects may exhibit no radiographic abnormalities if the shunt is small or nonspecific changes. The best indicator of the presence of a shunt lesion is pulmonary overcirculation seen as symmetrical enlargement of the pulmonary arteries and veins, an increased number of small vascular markings in the peripheral lung fields and a diffuse unstructured interstitial pattern.
Complex lesions can be challenging and may not be amenable to diagnosis from survey radiographs. One of the more common lesions is the tetralogy of Fallot, which is a combination of pulmonic stenosis, right ventricular hypertrophy, ventricular septal defect and overriding aorta. This combination of lesions may result in quite variable radiographic signs depending upon the severity of the anomalies. It is principally of interest as it may mimic an uncomplicated pulmonic stenosis.
Differential diagnosis of acquired cardiac disease
When evaluating radiographs for suspected acquired cardiac disease the same standard interpretation paradigm. The signalment of the patient is potentially helpful for formulating a reasonable differential diagnosis list. Valvular incompetence due to endocardiosis is most commonly a clinically significant lesion in older toy and small breed dogs. Both atrioventricular valves are affected but the mitral valve lesion is the one that typically produces clinical signs. As valvular incompetence worsens left atrial enlargement develops. This may cause a cough at night due to pressure on the left caudal lobar bronchus before any radiographic signs of failure are seen. The disease progresses to left ventricular dilation as the myocardium fails and pulmonary venous congestion and edema are seen in decompensated cases. Cardiogenic pulmonary edema due to mitral endocardiosis is first visible in the hilar zone of the lungs and spreads to the periphery. Rarely, edema may affect the right caudal lung lobe only. Tricuspid endocardiosis seldom causes purely right-sided cardiac failure but this is seen in late stage left side disease.
Acquired left sided cardiac disease in large and giant breed dogs is usually cause by dilated cardiomyopathy (DCM). The heart size may range from normal to severe generalized cardiomegaly. Cardiogenic edema is common and may have a rapid onset and is often quite severe. The distribution may be similar to that seen in mitral endocardiosis but often has a diffuse interstitial location or a caudodorsal interstitial and alveolar pulmonary appearance. Acute onset myocardial failure from mitral valve endocardiosis can result in a localized to the right caudal lung lobe.
The most common acquired cardiac disease of cats is hypertrophic cardiomyopathy (HCM). Radiographic changes include mild to severe left atrial enlargement and mild to moderate right atrial. Biatrial enlargement is seen as bulges at the right craniolateral (10 o'clock) and left borders (3 o'clock) of the heart on the VD or DV film that is described as having a "valentine heart" appearance. There is usually no alteration in the left ventricle's size or shape. Radiographic evidence of failure includes pulmonary venous congestion that is best appreciated in the hilar zone on the lateral film. Pulmonary edema appears as unstructured interstitial, alveolar or mixed patterns with a patchy random or perivascular distribution. Pleural effusion can be seen by itself or in combination with pulmonary edema when cats present in congestive heart failure.
Infestation by the heartworm Dirofilaria immitis occurs in both dogs and cats. Clinically normal or minimally affected animals usually have no appreciable radiographic changes. The mildest abnormalities which may be detected are enlargement of the mid and peripheral zone portions of the caudal lobar pulmonary arteries. Larger parasite burdens cause right sided cardiomegaly, main pulmonary artery enlargement and enlargement and tortuosity of the pulmonary arteries. Pulmonary infiltrate with eosinophilia (PIE) is a common allergic response to the heartworms. The radiographic features of PIE include a moderate to severe unstructured interstitial pattern, moderate to sever bronchial pattern and rarely interstitial nodules. This pattern may make it difficult to see the enlarged pulmonary arteries. PIE may also occur with a small parasite load with no cardiac or vascular changes.
Pulmonary thrombo-embolism (PTE) is a possible complication to heartworm infestation especially following adulticide treatment. Although HWD is the most common cause of PTE but it also occurs with many other diseases that result in hypercoagulable states and disseminated intravascular coagulation. The disease has a range of radiographic appearance. Radiographs may be completely normal which may explain why this disease is diagnosed more frequently post mortem than in the live patient. Radiographic abnormalities associated with PTE can include: focal or lobar hyperlucency due to absence of blood flow, focal pleural effusion or lobar alveolar lung patterns. Additionally, focal alveolar patterns are located at the periphery of the lungs and are wedge shaped in appearance with the apex of the lesion pointing toward the hilus while the base towards the pleural surface. Truncation (abrupt termination, or pruning) is the absence of normal branching of the peripheral pulmonary arteries. These changes are consistent with regional oligemia. Definitive diagnosis of PTE is difficult and often requires tests such as selective pulmonary arteriography, pulmonary perfusion scintigraphy or computed tomography angiography.
Pericardial Effusion. Accumulation of fluid within the pericardial sac may occur secondary to neoplasia, infection, and hemorrhage. However in some cases an underlying cause cannot be found (idiopathic). In pericardial effusions, the cardiac silhouette will become enlargement without specific vessel or chamber enlargement. The result is often a severe enlargement has been described as basketball or pumpkin like. DCM or atrioventricular valve dysplasia can cause similar severe enlargement. One characteristic feature is a very smooth arcing curve forming the caudodorsal border of the heart on the lateral film. Pericardial effusion results in right heart failure and pleural fluid accumulation that may partly obscure the outline of the heart. Congenital peritoneopericardial hernias may mimic pericardial effusion. The lack of normal development of the diaphragm results in a continuum between the pericardial and peritoneal cavities allows abdominal organs to pass into the pericardium. These hernias are often clinically silent and are discovered by accident. The cardiac silhouette is severely enlarged, abnormally shaped, and of can be of inhomogeneous opacity due to the presence of omental and mesenteric fat and gas filled small intestinal loops if herniated into the pericardial space. Portions of the gastrointestinal tract may be found within the pericardium and occasionally animals present with acute onset intestinal obstruction due to incarceration of the hernia. Echocardiography is required for definitive diagnosis of pericardial disease. The most causes of pericardial effusion in the dog are secondary to a right atrial hemangiosarcoma or heart base tumor (chemodectoma most common). Idiopathic hemorrhagic effusions can be seen in large breed dogs. Small breed dogs with chronic endocardiosis can develop a hemorrhagic effusion because of acute left atrial tears.
The Caudal Mediastinum.
Abnormalities of the caudal mediastinum have been covered in the section of the esophagus. Several gastro-esophageal abnormalities should be mentioned however. This includes paraesophageal hernias, hiatal or sliding esophageal hernia and a gastroesophageal intussusception, (Figure 87). These abnormalities can be seen as discrete soft tissue mass effect in the caudal esophagus with widening of the caudal mediastinum being present on the VD radiograph. Left lateral radiographs will aid in documenting a sliding hernia more readily than the right lateral radiograph. The Sharpei breed is predisposed to gastroesophageal hernias. Gastro-esophageal intussusceptions are rare and are characterized by a soft tissue and gas filled mass in the caudal esophagus. Gastric rugal folds in the esophagus can be identified characteristic of this abnormality. As with other esophageal disorders, aspiration pneumonia can be present. Esophageal masses can be located at the gastroesophageal junction. Caudodorsal mediastinal masses can originate in and around any of the soft tissues of the caudal mediastinum. These tumors are rare in the dog and cat.
Summary
Thoracic radiography is a main stay in the initial work up and evaluation in cats with dyspnea. Cardiopulmonary disorders are at the top of the list for rule outs, except in older cats where neoplasia becomes a higher differential consideration depending on the thoracic radiographic features. Initial DV radiography can be done to rule out certain abnormalities (pneumothorax, pleural effusion); however, follow up three view thorax would be required for complete evaluation of the thorax. In addition, a lateral radiograph of the pharynx and skull may be required in order to identify any reasons for dyspnea associated with the upper airways.
Suggested Reading/References
Thrall, DE. Veterinary Diagnostic Radiology, 5th Edition. WB Saunders: 2008.
Dennis R, Kirberger RM, Wrigley RH and Barr FJ. Handbook of Small Animal Radiological Differential Diagnosis, WB Saunders, 2001.
Schwarz T and Johnson V. BSAVA Manual of Canine and Feline Thoracic Imaging. BSAVA, 2008.
Luis Fuentes V and Swift S. Manual of Small Animal Cardiorespiratory Medicine and Surgery. BSAVA, 1998.
King L. Textbook of Respiratory Diseases in the Dog and Cat. WB Saunders, 2005.