Targeting antimicrobials in food animals (part 2) (Proceedings)
These proceedings present data related to the question of how long to wait after administering a single injection antimicrobial before applying success/failure criteria. More accurately, we will evaluate success/failure and mortality data based on administering a uniform regimen and then waiting different periods before applying success/failure criteria, and the animal subsequently being eligible for further therapy.
These proceedings present data related to the question of how long to wait after administering a single injection antimicrobial before applying success/failure criteria. More accurately, we will evaluate success/failure and mortality data based on administering a uniform regimen and then waiting different periods before applying success/failure criteria, and the animal subsequently being eligible for further therapy. This is quite different from administering the drug for differing periods of time and evaluating clinical outcome.
Data concerning the outcome of treatment following differing periods of application of the same repeated regimen are extremely rare. The current (and long-standing practice) of treating for an extended period beyond clinical cure to minimize relapses is being recognized as having a downside related to continued antimicrobial exposure of normal flora and any resistant pathogen subpopulation. In my opinion, the most critically needed antimicrobial use data are those that that will help us to balance minimization of treatment failures and relapses while at the same time minimizing resistance selection pressure.
Single injection, long duration antimicrobial therapy for veterinary use was first available for cattle; long-acting, 200 mg/ml oxytetracycline became available in the mid 1970s. Recently this type of therapeutic approach became available for dogs and cats with a cephalosporin demonstrating T ½ values of 133 hours in dogs and 166 hrs in cats (cefovecin, Convenia™, Pfizer Animal Health). Most recently, ceftiofur crystalline free acid (Excede™, Pfizer Animal Health) has been labeled for horses with two intramuscular injections administered 4 days apart, reported to maintain therapeutic concentrations against Streptococus equi ssp. Zooepidemicus for 10 days..
The question is particularly relevant when you consider the large number of single-injection antimicrobials available for cattle.
• Ceftiofur crystalline free acid (Excede®, Pfizer)
• Enrofloxacin (Baytril®, Bayer)
• Florfenicol (Nuflor®, Schering-Plough)
• Tilmicosin (Micotil®, Elanco)
• Tulathromycin (Draxxin®, Pfizer)
• Oxytetracycline 200 mg/ml (Liquamycin LA-200®, Pfizer, and generics)
• Oxytetracycline 300 mg/ml (Tetradure®, Merial)
In addition, there are antimicrobials with cattle labels indicating administration 48 hours apart as either one of the label regimens or the sole label regimen.
• Ceftiofur hydrochloride (Excenel® RTU, Pfizer)
• Danofloxacin (A-180®, Pfizer)
Post-treatment intervals for antimicrobials used in food animals
One method of looking at post-treatment intervals is to look at the individual drug pharmacokinetics along with susceptibility information (or susceptible breakpoints from susceptibility testing) and look at when serum concentrations (or tissue concentrations?) fall below the cutoff point. Table 1 gives suggested PTIs developed by the author using this method. Question marks indicate special cases where available clinical data merits additional discussion. In addition, the 3 studies following table 1 provide clinical trial data evaluating different post-treatment intervals for commonly used single-injection antimicrobials used in the therapy of bovine respiratory disease. These studies shed some light on the effects of waiting longer to determine success or failure in relation to respiratory disease.
Table 1: Suggested post-treatment intervals for antimicrobials used in cattle
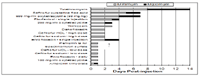
Adapted, and modified from: Apley MD. Bovine Respiratory Disease: Pathogenesis, Clinical Signs, and Treatment in Lightweight Calves. in The Veterinary Clinics of North America, Food Animal Practice, Stocker Cattle Management, W. B. Saunders, Philadelphia, (22:2). 2006
Studies evaluating different post-treatment intervals
The following studies describe the effects of waiting different periods of time prior to applying success/failure criteria to cattle treated for respiratory disease in field settings. Studies 1 and 3 are taken from pivotal approval data submitted for approval of these drugs. Study 2 describes a situation where success/failure criteria were applied at periods longer than would be conventionally applied in today's management schemes.
1. Tulathromycini
Crossbred heifer feeder calves with a mean BW of 473 lbs (range 324 to 640 lbs) originating from MO, AR, KY, and TN were utilized in the study. Entrance into the study required a combination of a clinical condition score of 1, 2, of 3 on a 0 – 4 scale combined with a rectal temperature ≥ 104.0° F, followed by label administration of tulathromycin (Draxxin®, Pfizer Animal Health). Cattle meeting entrance criteria were randomly assigned to either a 7, 10, or 14 day post-treatment interval before they were eligible for further treatment. The results of the study are summarized in the following table.
Summary of Results Through Day 56 of Study

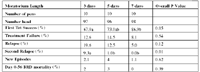
2. Tilmicosin
Crossbred steer calves weighing from 402 to 706 lbs from CO, KS, OK and WY were enrolled in this study conducted in Wellington, CO. Animals with a clinical score of ≥ 2 on a scale of 1 – 5 combined with a rectal temperature of ≥ 104.0° F were admitted to the study and treated with tilmicosin phosphate (Micotil®, Elanco Animal Health) according to label directions. Cattle were randomly assigned to treatment groups consisting of 3-, 5-, or 7-day post-treatment intervals.
Study results are reported in the table below
3. Ceftiofur Crystalline Free Acid
Three feedlots participated in this study. When cattle were pulled from the home pen for clinical signs of BRD (abnormal respiration and depression), and had a rectal temperature of ≥ 104.0° F, they were treated with ceftiofur crystalline Free Acid (Excede™, Pfizer Animal Health) according to label directions. The treated cattle were allocated to post-treatment intervals of 3-, 5- or 7- days.
Values in a row with different superscripts are statistically different (P<0.05)

1. Percentage of cattle that did not qualify for retreatment on the first day they were eligible (the first day after the PTI)
2. Percentage of cattle that never qualified for retreatment during the study.
3. Percentage of cattle that were retreated between the first day they were eligible, but before day 9, and never qualified for additional treatment during the study.
4. Dead and other animals removed from the study were not included in the analysis.
In my opinion, these studies suggest that multiple factors come into play when determining how long to wait before applying success/failure criteria. These involve duration of activity of the antimicrobial (which may or may not be primarily dependant on the concentrations we happen to be measuring) and also time to clinical recovery of animals after the insulting pathogen has been eliminated or suppressed.