Sweet success: Managing the difficult diabetic patient (Sponsored by Purina Veterinary Diets)
The management of newly diagnosed and complicated cases of diabetes in cats and dogs

Management of diabetes mellitus in small animals can be challenging; however, the application of a systematic method of dietary and pharmaceutical (insulin, oral hypoglycemics) interventions coupled with practical monitoring strategies can result in improved patient care and client satisfaction. This article will cover the management of newly diagnosed and complicated cases of diabetes in cats and dogs.
Feline diabetes mellitus
Diagnosis of feline diabetes mellitus
Feline diabetes is most often type 2 in nature1 and is one of the most common endocrinopathies of the cat.2,3 Middle-aged to older, male cats with a history of obesity are most at risk.4 Clinical signs of type 2 diabetes mellitus are subtle and progressive over a period of months to years until blood glucose rises above the renal threshold, which is quite high in the cat, and polyuria/polydipsia ensues. Obesity combined with fasting or postprandial hyperglycemia may be the only clinical “sign” of early type 2 diabetes mellitus. Cats affected with diabetic neuropathy will have trouble jumping onto high surfaces such as counters and beds because the hind limbs are usually affected.5 As blood glucose concentrations exceed the renal threshold (which may be as high as 350 mg/dl in some cats), polyuria and secondary polydipsia become the primary clinical signs. Weight loss begins to occur as a result of insulin resistance and insulin deficiency with calories lost in glucose-laden urine.
In cats, physical examination findings of uncomplicated diabetes mellitus are typically nonspecific. The most common physical examination findings in cats are lethargy and depression, dehydration, unkempt haircoat, hepatomegaly, and muscle wasting. Plantigrade rear limb stance resulting from diabetic neuropathy is observed in approximately 10% of diabetic cats in previous studies.6 However, recent evidence suggests that diabetic cats can have a variety of clinical signs suggestive of diabetic neuropathy including pain on palpation of distal extremities, hypersensitivity, gait abnormalities, and palmagrade stance.7 Based on electromyogram (EMG), nerve biopsy, and nerve conduction studies, all diabetic cats have some degree of diabetic neuropathy at the time of presentation.8
A diagnosis of diabetes mellitus should be based on the presence of clinical signs compatible with diabetes mellitus and evidence of fasting hyperglycemia and/or glycosuria. Common clinicopathologic features of diabetes mellitus in cats include fasting hyperglycemia, hypercholesterolemia, increased liver enzymes (ALP, ALT), neutrophilic leukocytosis, proteinuria, increased urine specific gravity, azotemia, and glycosuria.5
Many cats are susceptible to stress-induced hyperglycemia in which the serum glucose concentrations approach 300 to 400 mg/dl. Serum fructosamine is formed by glycosylation of serum protein such as albumin. The fructosamine concentration in serum is directly related to blood glucose concentration. However, because albumin has a shorter life span than hemoglobin has, fructosamine concentrations reflect more recent (one to three weeks) changes in serum glucose concentrations than glycosylated hemoglobin does. Measuring serum fructosamine concentrations in cats may be of benefit when trying to distinguish early or subclinical diabetes mellitus from stress-induced hyperglycemia.9 One study of 17 normal cats showed that transient glucose administration (1 g/kg 50% glucose solution, intravenously) did not increase serum fructosamine concentrations.10 In the same study, it was noted that 50% of sick cats showed mild increases in serum fructosamine concentrations consistent with subclinical to mildly clinical diabetes mellitus even when the serum glucose concentrations were in the normal or near normal range.10 This finding suggests that subclinical type 2 diabetes mellitus is probably much more common than previously believed and that obese cats with increased serum fructosamine levels should be followed carefully for evidence of developing diabetes mellitus. In cats, normal fructosamine concentrations are 283 +/- 32 μmol/L.
Management of newly diagnosed diabetic cats
The management of newly diagnosed feline diabetic patients includes first and foremost attention to diet (Table 1). Initial studies using a canned high-protein/low-carbohydrate diet and the starch blocker acarbose have shown that 58% of cats were able to discontinue insulin injections and those with continued insulin requirements could be regulated with a much lower dosage (1 U b.i.d.).11 Comparison of canned high-fiber/moderate-carbohydrate diets versus low-carbohydrate/low-fiber diets showed that cats fed low-carbohydrate diets were almost twice as likely to discontinue insulin injections.12 Feeding newly diagnosed diabetic cats a canned low-carbohydrate, high-protein diet, such as Purina Veterinary Diets® DM Dietetic Management®, will result in the highest remission rates. For cats that refuse to eat canned food, Purina DM dry combined with acarbose (12.5 mg b.i.d. with food) is another alternative.
Table 1. Nutrient (Macro) Composition of Feline Therapeutic Diets for Diabetes Mellitus
% Dry Matter
Food Form
Protein
Carbohydrates
Fat
Crude Fiber
Hill's Prescription Diet
Feline m/d
Canned
52.8
15.7
19.4
6.0
Feline m/d
Dry
51.1
15.1
21.8
6.0
Purina Veterinary Diets
DM Dietetic Management
Canned
53.4
4.5
32.9
2.9
DM Dietetic Management
Dry
57.9
14.9
17.9
1.3
Royal Canin Veterinary Diet
Diabetic
Dry
46.1
25.3
12.0
4.81
Diabetic
Canned
57.3
15.3
16.4
7.6
Studies have shown that compared with PZI and Lente insulin, glargine insulin is associated with the highest remission rates.13 Yet veterinarians often balk at the cost of this human recombinant insulin. One strategy to reduce the cost of this insulin and to improve the administration technique is to use an insulin injector pen. The cost of the 3-cc cartridge and pen is about one-tenth the cost of a 10-cc bottle of glargine insulin. Another option would be to use PZI insulin, which is associated with a lower remission rate (70%) but is easier to administer and less costly.14,15 In either case, in my experience, an initial dosage of 2 U per cat twice daily has not been associated with hypoglycemia. For owners who prefer to give oral medication, glipizide, a sulfonylurea antidiabetic agent, has been used to successfully treat diabetes mellitus in cats at a dosage of 2.5 mg twice daily when combined with a high-protein, lowcarbohydrate diet.16
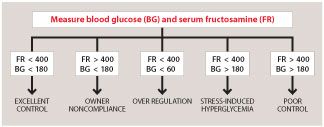
Figure 1. Interpretation of blood glucose and serum fructosamine measurements to monitor diabetic dogs and cats.Monitoring newly diagnosed and treated feline diabetics can be challenging. I prefer to use a slow method of remission induction using urine glucose monitoring at home coupled with periodic (every three weeks) serum chemistry, urinalysis, and fructosamine monitoring. An algorithm for monitoring with serum fructosamine is shown in Figure 1. The owner must agree to not increase the insulin dosage based on urine glucose, and to only decrease the dosage if the urine glucose becomes negative. Owners are not encouraged to monitor cats using blood glucose testing because the stress of a new diet, administration of insulin, and altered home routine make home glucose curves difficult to interpret.17 Continuous glucose monitors may be more effective in determining if the cat is going into remission or if hypoglycemia is occuring.18-20
Complicated feline diabetes
One of the most common reasons for a cat to fail to go into diabetic remission is a concurrent endocrinopathy. In cats, the most common endocrinopathy associated with diabetes mellitus is hypersomatotropism.21,22 Acromegaly should be suspected in male cats that do not go into remission or that require increasing dosages of insulin to control their diabetes.Other clinical signs of acromegaly include stridor and increased interdental spaces. Diagnosis is achieved by measuring insulin-like growth factor 1 concentrations.21 Confirmation of a pituitary adenoma may be made using MRI or CT.23 If a cat is diagnosed with acromegaly, the options for treatment include radiation treatment of the pituitary tumor or cryohypophysectomy/hypophysectomy.24,25 New medical therapy for acromegaly may become available in the future, but at present, it is very expensive.
The second most common endocrinopathy found in diabetic cats is hyperthyroidism26; about 10% of diabetic cats may have concurrent hyperthyroidism. Because of the wide fluctuations in serum thyroid concentrations, hyperthyroid cats will be difficult to manage. However, permanent treatment of the hyperthyroidism using iodine-131 (131I) will result in remission in about 50% of the cases.
Finally, diabetic cats with concurrent hyperadrenocorticism may be particularly difficult to manage. Hyperadrenocorticism should be suspected in female cats with insulin-resistant diabetes mellitus, persistent polydipsia and polyuria, alopecia, and fragile skin.27 Diagnosis can be suspected on the basis of a lack of suppression during a low-dose dexamethasone suppression test, urinary excretion of glucocorticoids, and/or elevated precursors of endogenous ACTH concentrations.27-29
Renal complications of diabetes mellitus are common in human diabetics and may also be common in cats.30,31 The earliest sign of diabetic nephropathy is microalbuminuria followed by an increased protein:creatinine ratio in the urine. Azotemia is a late consequence of diabetic nephropathy; however, with good regulation, it may be reversible. Improved diabetic regulation using a canned low-carbohydrate diet and insulin or oral hypoglycemic agents is most successful in treating diabetic nephropathy. Feeding canned food is particularly important in diabetic cats with concurrent renal disease in order to prevent dehydration and secondary renal damage.
Diabetic cats often have concurrent gastrointestinal disease, such as oral disease, pancreatitis, inflammatory bowel disease, and cholangiohepatitis.32 Some of these gastrointestinal signs may originate as a result of suppressed immune function (periodontitis); autonomic diabetic neuropathy (vomiting, diarrhea); or possibly as a result of concurrent pancreatitis, cholangiohepatitis, or inflammatory bowel disease. Careful attention to diet (low-carbohydrate Purina Veterinary Diets® DM Dietetic Management® or Purina Veterinary Diets® EN Gastroenteric® Feline Formula), good oral health, the addition of probiotics (e.g. FortiFlora® Feline Probiotic Nutritional Supplement), and judicious use of antibiotics and steroids (for pancreatitis) may all help when managing a cat with concurrent gastrointestinal disease and diabetes.33
Canine diabetes mellitus
Diagnosis of diabetes mellitus in dogs
Insulin-dependent diabetes mellitus is a diabetic state in which endogenous insulin secretion is never enough to prevent ketone production. Type 1 diabetes mellitus is a diabetic state in which insulin secretion may be reduced or absent and which is readily corrected by exogenous insulin. In dogs, type 1a diabetes is caused by autoimmune destruction of the beta cells (genetic disorder related to hypothyroidism), and Type 1b (mature onset diabetes of the young) is a non-immune destruction of islets caused by pancreatitis or an unknown cause.1
Dogs that have diabetes mellitus range in age from 4 to 14 years, with a peak incidence at 7 to 9 years. A genetic basis for diabetes mellitus type 1a is suspected in Samoyeds, Keeshonden, Labrador retrievers, and various terriers (Australian, Tibetan, Cairn). Other breeds commonly affected are miniature and toy poodles, dachshunds, beagles, puliks, miniature schnauzers, and miniature pinschers. Most dogs with diabetes mellitus present with the classic clinical signs of polyuria and polydipsia.5 Weight loss and polyphagia are commonly observed in dogs. Dogs with diabetes mellitus are also commonly presented with acute onset of blindness caused by bilateral cataracts. In dogs, dehydration and muscle wasting or thin body condition are the most common physical examination findings. Hepatomegaly is usually observed, and cataracts are observed in about 40% of diabetic dogs.5
A diagnosis of diabetes mellitus should be based on the presence of clinical signs compatible with diabetes mellitus and evidence of fasting hyperglycemia and glycosuria. Common clinicopathologic features of diabetes mellitus in dogs include fasting hyperglycemia, hypercholesterolemia, increased liver enzymes (ALP, ALT), neutrophilic leukocytosis, proteinuria, increased urine specific gravity, bacteriuria, and glycosuria.5
Management of canine diabetes mellitus
For diabetic dogs, the protein content of the diet should be normal or increased, particularly if the dog is obese. Higher protein formulations may also benefit underweight diabetic dogs with significant muscle wasting or those experiencing concurrent exocrine pancreatic insufficiency. A moderately carbohydrate-restricted diet (< 30% metabolizable energy [ME]) is recommended, and meals should have consistent carbohydrate content (Table 2).34 Low glycemic index carbohydrates such as sorghum and barley are recommended. Readily absorbed carbohydrates such as rice and corn syrup have high glycemic indices and should be avoided. Foods with high sugar content (semimoist foods) should not be used to improve the palatability of food prescribed for diabetic dogs. Diets with moderate dietary fiber content are recommended for diabetic dogs especially those that are overweight (crude fiber maximum 10% to 15% dry matter).
Table 2. Macronutrient Content of Selected Therapeutic Dog Foods Suitable for Diabetic Dogs
% Dry Matter
Form
Protein
Carbohydrates
Fat
Crude Fiber
Hill's Prescription Diets
Canine r/d
Canned
25.3
39.2
8.6
21.2
Canine r/d
Dry
34.6 38.2 8.2 13.1
Canine w/d
Canned
17.9 52.6 12.7 12.4
Canine w/d
Dry
19.2 50.8 8.7 16.4
Purina Veterinary Diets
DCO Dual Fiber Control
Dry 24.5 48.4 12.9 7.3
OM Overweight Management
Canned 44.2 21.0 10.5 18.5
OM Overweight Management
Dry 31.1 44.2 7.2 10.3
Royal Canin Veterinary Diet
Diabetic
Dry 40.7 31.8 13.2 8.8
Diabetic
Canned 53.5 19.3 22.6 16.6
Iams Veterinary Formula
Glucose and Weight Control Plus
Dry
29.1 50.4 9.6 3.2
Exercise, if prescribed and regular, may reduce insulin requirements in dogs. The owners should be instructed to walk the dog daily and avoid intermittent episodes of strenuous exercise, such as racing or hiking.
Human recombinant insulin is the most available insulin preparation on the market and is perfectly acceptable as insulin therapy for all dogs. Intermediate-acting insulin (NPH, Lente) or long-acting glargine is administered twice daily in dogs, at a starting dosage of 0.4 to 0.5 U/kg.35 In dogs, the long-acting insulin PZI is administered once or twice daily at 0.8 to 0.9 U/kg.36 Detemir insulin is dosed at 0.1 to 0.2 U/kg twice daily in dogs and may be the insulin of choice for larger dogs.37 To mimic the physiologic release of insulin, insulin should ideally be given with each meal. If the animal does not eat, the insulin dosage can be reduced (usually by one-half) or skipped entirely and the animal evaluated by the veterinarian to determine the cause of the anorexia.
In general, the client should be instructed to monitor the insulin effect and gross regulation of hyperglycemia by noting changes in appetite, attitude, body condition,polydipsia, polyuria, and urine glucose and ketone levels.38 Consistently high urine glucose readings coupled with uncontrolled clinical signs, such as polyuria/polydipsia, indicate that the insulin dose may be inadequate. Consistently negative readings on urine glucose may indicate that insulin dosages are either adequate or excessive. The veterinarian should assess diabetic dogs every three or four weeks during initial insulin therapy using serum fructosamine (Figure 1) and a serum chemistry profile. Blood glucose curves are not generally helpful in determining insulin dosage because of day-to-day variability.39,40 If a dog fails to respond to insulin and dietary therapy, an investigation into underlying causes is warranted and a continuous glucose monitor may be required.41
Complicated diabetes mellitus in dogs
Hypothyroidism is a common concurrent endocrinopathy in diabetic dogs. The genetic abnormality associated with type 1 diabetes mellitus in dogs is also associated with lymphocytic thyroiditis in dogs.42,43 A diagnosis can be made identifying a low or low normal thyroxine concentration and a high endogenous thyroid-stimulating hormone concentration. Hyperadrenocorticism, if present in a diabetic dog, usually precedes the diagnosis of diabetes mellitus as long-standing, poorly treated or untreated hyperadrenocorticism can result in secondary diabetes mellitus from insulin resistance.43 Treatment of the hyperadrenocorticism using mitotane has the best success in my experience.
Concurrent pancreatitis or exocrine pancreatic insufficiency (EPI) is common in diabetic dogs. About 40% of diabetic dogs have a history of pancreatitis, and about 10% may have EPI. Screening newly diagnosed diabetics for both pancreatitis and EPI should be done at the time of diagnosis and again if poor diabetic regulation is encountered. Serum canine pancreatic lipase immunoreactivity (cPLI) and trypsin-like immunoreactivity (TLI) tests are sufficient for screening a dog initially. Pancreatitis should be suspected in any diabetic dog that presents with acute onset of vomiting or abdominal pain. EPI is usually subtle; intermittent anorexia and hypoglycemia is more commonly seen than diarrhea or steatorrhea. Treatment with exogenous pancreatic enzymes is recommended if EPI is identified.
Hyperlipidemias are commonly seen in diabetic dogs and may be a consequence of diabetes mellitus, pancreatitis, hypothyroidism, or hereditary hyperlipidemic syndromes (e.g. miniature schnauzers). Dietary fat restriction (< 12% on a dry matter basis or < 30% ME) is recommended for diabetic dogs with concurrent chronic pancreatitis or persistent hypertriglyceridemia (miniature schnauzers). Foods with a high fat content should not be given to improve the palatability of food prescribed for diabetic dogs. Instead, the low-carbohydrate, low-fat food may be made more palatable with agents such as warm low-fat chicken or beef broth.
Conclusion
The diagnosis and management of diabetes mellitus in cats and dogs is an important aspect of the practice of small animal internal medicine. The purpose of this article was to share some of my experiences in regulating diabetic animals. Clear direction, expectations, and followup about the care of their diabetic pet will help reduce owners' stress and improve the quality of life for these patients.44
References
1. Rand JS, Fleeman LM, Farrow HA, et al. Canine and feline diabetes mellitus: nature or nurture? J Nutr 2004;134(8 Suppl):2072S-2080S.
2. Rand JS. Understanding feline diabetes: pathogenesis and management. Vet Q 1998;20 Suppl 1:S35-S37.
3. Rand JS. Feline diabetes. Vet Clin North Am Small Anim Pract 2013;43:xi-xii.
4. Rand JS. Pathogenesis of feline diabetes. Vet Clin North Am Small Anim Pract 2013;43:221-231.
5. Greco DS. Diagnosis of diabetes mellitus in cats and dogs. Vet Clin North Am Small Anim Pract 2001;31(5):845-53, v-vi.
6. Reusch C. Feline diabetes mellitus. In: Ettinger, SJ, Feldman, EC, eds. Textbook of veterinary internal medicine. 7th ed. (Vol. 2). St. Louis, Mo: Saunders Elsevier, 2010;1800.
7. Munana KR. Long-term complications of diabetes mellitus, Part I: Retinopathy, nephropathy, neuropathy. Vet Clin North Am Small Anim Pract 1995;25:715-730.
8. Mizisin AP, Shelton GD, Burgers ML, et al. Neurological complications associated with spontaneously occurring feline diabetes mellitus. J Neuropathol Exp Neurol 2002;61(8):872-884.
9. Crenshaw KL, Peterson ME, Heeb LA, et al. Serum fructosamine concentration as an index of glycemia in cats with diabetes mellitus and stress hyperglycemia. J Vet Intern Med 1996;10(6):360-364.
10. Lutz TA, Rand JS, Ryan E. Fructosamine concentrations in hyperglycemic cats. Can Vet J 1995;36(3):155-159.
11. Mazzaferro EM, Greco DS, Turner AS, et al. Treatment of feline diabetes mellitus using an alpha-glucosidase inhibitor and a low-carbohydrate diet. J Feline Med Surg 2003;5(3):183-189.
12. Bennett N, Greco DS, Peterson ME, et al. Comparison of a low carbohydrate-low fiber diet and a moderate carbohydrate-high fiber diet in the management of feline diabetes mellitus. J Feline Med Surg 2006;8(2):73-84.
13. Bloom CA, Rand J. Feline diabetes mellitus: clinical use of long-acting glargine and detemir. J Feline Med Surg 2014;16(3):205-215.
14. Nelson RW, Henley K, Cole C. Field safety and efficacy of protamine zinc recombinant human insulin for treatment of diabetes mellitus in cats. J Vet Intern Med 2009;23(4):787-793.
15. Norsworthy G, Lynn R, Cole C. Preliminary study of protamine zinc recombinant insulin for the treatment of diabetes mellitus in cats. Vet Ther 2009;10(1-2):24-28.
16. Palm CA, Feldman EC. Oral hypoglycemics in cats with diabetes mellitus. Vet Clin North Am Small Anim Pract 2013;43(2):407-415.
17. Alt N, Kley S, Haessig M, et al. Day-to-day variability of blood glucose concentration curves generated at home in cats with diabetes mellitus. J Am Vet Med Assoc 2007;230(7):1011-1017.
18. Moretti S, Tschuor F, Osto M, et al. Evaluation of a novel real-time continuous glucose-monitoring system for use in cats. J Vet Intern Med 2010;24(1):120-126.
19. Surman S, Fleeman L. Continuous glucose monitoring in small animals. Vet Clin North Am Small Anim Pract 2013;43(2):381-406.
20. Dietiker-Moretti S, Muller C, Sieber-Ruckstuhl N, et al. Comparison of a continuous glucose monitoring system with a portable blood glucose meter to determine insulin dose in cats with diabetes mellitus. J Vet Intern Med 2011;25(5):1084-1088.
21. Berg RI, Nelson RW, Feldman EC, et al. Serum insulin-like growth factor-I concentration in cats with diabetes mellitus and acromegaly. J Vet Intern Med 2007;21(5):892-898.
22. Greco DS. Feline acromegaly. Top Companion Anim Med 2012;27(1):31-35.
23. Lamb CR, Ciasca TC, Mantis P, et al. Computed tomographic signs of acromegaly in 68 diabetic cats with hypersomatotropism. J Feline Med Surg 2014;16(2):99-108.
24. Sellon RK, Fidel J, Houston R, et al. Linearaccelerator-based modified radiosurgical treatment of pituitary tumors in cats: 11 cases (1997-2008). J Vet Intern Med 2009;23(5):1038-1044.
25. Blois SL, Holmberg DL. Cryohypophysectomy used in the treatment of a case of feline acromegaly. J Small Anim Pract 2008;49(11):596-600.
26. Crenshaw KL, Peterson ME. Pretreatment clinical and laboratory evaluation of cats with diabetes mellitus: 104 cases (1992-1994). J Am Vet Med Assoc 1996;209(5):943-949.
27. Chiaramonte D, Greco DS. Feline adrenal disorders. Clin Tech Small Anim Pract 2007;22(1):26-31.
28. Goossens MM, Meyer HP, Voorhout G, et al. Urinary excretion of glucocorticoids in the diagnosis of hyperadrenocorticism in cats. Domest Anim Endocrinol 1995;12(4):355-362.
29. Benchekroun G, de Fornel-Thibaud P, Dubord M, et al. Plasma ACTH precursors in cats with pituitary-dependent hyperadrenocorticism. J Vet Intern Med 2012;26(3):575-581.
30. Bloom CA, Rand JS. Diabetes and the kidney in human and veterinary medicine. Vet Clin North Am Small Anim Pract 2013;43(2):351-365.
31. Al-Ghazlat SA, Langston CE, Greco DS, et al. The prevalence of microalbuminuria and proteinuria in cats with diabetes mellitus. Top Companion Anim Med 2011;26(3):154-157.
32. Diehl KJ. Long-term complications of diabetes mellitus, part II: gastrointestinal and infectious. Vet Clin North Am Small Anim Pract 1995;25(3):731-751.
33. Armstrong PJ, Williams DA. Pancreatitis in cats. Top Companion Anim Med 2012;27(3):140-147.
34. Fleeman LM, Rand JS, Markwell PJ. Lack of advantage of high-fibre, moderate-carbohydrate diets in dogs with stabilised diabetes. J Small Anim Pract 2009;50(11):604-614.
35. Hess RS, Drobatz KJ. Glargine insulin for treatment of naturally occurring diabetes mellitus in dogs. J Am Vet Med Assoc 2013;243(8):1154-1161.
36. Maggiore AD, Nelson RW, Dennis J, et al. Efficacy of protamine zinc recombinant human insulin for controlling hyperglycemia in dogs with diabetes mellitus. J Vet Intern Med 2012;26(1):109-115.
37. Gilor C, Graves TK. Synthetic insulin analogs and their use in dogs and cats. Vet Clin North Am Small Anim Pract 2010;40:297-307.
38. Briggs CE, Nelson RW, Feldman EC, et al. Reliability of history and physical examination findings for assessing control of glycemia in dogs with diabetes mellitus: 53 cases (1995-1998). J Am Vet Med Assoc 2000;217(1):48-53.
39. Fleeman LM, Rand JS. Evaluation of day-to-day variability of serial blood glucose concentration curves in diabetic dogs. J Am Vet Med Assoc 2003;222(3):317-321.
40. Casella M, Wess G, Hassig M, Reusch CE. Home monitoring of blood glucose concentration by owners of diabetic dogs. J Small Anim Pract 2003;44(7):298-305.
41. Wiedmeyer CE, DeClue AE. Continuous glucose monitoring in dogs and cats. J Vet Intern Med 2008;22(1):2-8.
42. Catchpole B, Kennedy LJ, Davison LJ, et al. Canine diabetes mellitus: from phenotype to genotype. J Small Anim Pract 2008;49(1):4-10.
43. Blois SL, Dickie E, Kruth SA, et al. Multiple endocrine diseases in dogs: 35 cases (1996-2009). J Am Vet Med Assoc 2011;238(12):1616-1621.
44. Niessen SJ, Powney S, Guitian J, et al. Evaluation of a quality-of-life tool for dogs with diabetes mellitus. J Vet Intern Med 2012;26(4):953-961.
Episode 67: Choosing trusted supplements
October 20th 2021In this episode of The Vet Blast Podcast, Dr Adam Christman chats with Dr Janice Huntingford about the latest insights into selecting the best supplements for your patients, including the importance of recommending and utilizing products that have a substantial amount of science and research behind them. (Sponsored by Vetoquinol)
Listen