How much does it take? Antimicrobials and dosing strategies in food animals (Proceedings)
There are well thought out and clinically confirmed label regimens for label indications. For example, the antimicrobials labeled for individual animal treatment of respiratory disease in cattle and swine have label regimens which most likely give you the majority of the clinical results you will get.
Label regimens
There are well thought out and clinically confirmed label regimens for label indications. For example, the antimicrobials labeled for individual animal treatment of respiratory disease in cattle and swine have label regimens which most likely give you the majority of the clinical results you will get. When we get into enteric disease, labels are very few, and we often end up looking for efficacy in extra-label use. The problem is that confirmed antimicrobial efficacy in food animal enteric disease is very rare, and we end up looking at in-vitro minimal inhibitory concentrations for which there are no approved veterinary breakpoints.
Combination antimicrobial therapy
There are some in-vitro studies showing synergism for food animal pathogens, but they are few and far between. I have been unable to find clinical efficacy studies demonstrating improved clinical response to a combination of antimicrobials in food animals. It appears that this practice is still relatively common, in cattle anyway, with acceptance and promotion based on anecdotal reports. I am not a believer in antimicrobial combinations for diseases such as bovine respiratory disease. This presentation will focus on individual antimicrobial regimens; if you just want to believe and go ahead with antimicrobial combination therapy, you are on your own as far as supporting data.
Going off label
When we are off label, our best bet is to understand the pharmacokinetics /pharmacodynamics (PK/PD) of the antimicrobials we are using and adjust regimens accordingly. This is not a perfect approach, and is always trumped by clinical data. However, the PK/PD approach is worthy in that we can rule out some completely inappropriate clinical strategies.
There are some drugs where we likely won't be increasing dosing regimens due to cost, and some where we cannot legally use them off-label (e.g., fluoroquinolones in food animals, sulfas in lactating dairy cattle, any antimicrobial in feed). In contrast, there are some classes where properties of the drugs (cost, withdrawal time, safety, spectrum) make them ideal candidates for extra-label regimens. We will focus on two, oxytetracycline, and ceftiofur.
Oxytetracycline:
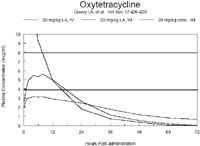
Comparing oxytetracycline preparations in cattle
Pharmacodynamics for oxytetracycline, chlortetracycline, and tetracycline are not well established for any pathogen. It is assumed that they are time > MIC or AUC:MIC dependant because they are bacteriostatic, but this is not confirmed, especially for veterinary pathogens.
Susceptibility testing
Tetracycline breakpoints adapted from CLSI M31-A3. The "generic" breakpoints have been derived utilizing veterinary pharmacokinetic data combined with pharmacodynamic data derived from other bacterial species and without supporting clinical data.

In addition to direct antimicrobial activity, the tetracyclines have demonstrated other therapeutic effects. Extensive effects of tetracyclines on inflammation have been established, many of which have been utilized in human dermatologyi These include suppression of neutrophil chemotaxis, inhibition of T-lymphocyte activation and phospholipase A2, and inhibition of mitogen-induced human lymphocytic proliferation. While the use of tetracyclines for acne was initially thought to be based on inhibition of Propionibacterium acnes, it is now believed that much of the therapeutic benefit is actually due to the inhibition of P. acnes lipase, neutrophil chemotaxis, and proinflammatory cytokines and metaloproteinases. It is tempting to speculate that some of the effects we see from lower systemic concentrations resulting from in-feed tetracyclines may be related to activities such as these.
Putting the in-feed tetracyclines in perspective related to injectable tetracyclines is not that easy, especially with more limited data available. Plasma concentrations from the administration of chlortetracycline in feed have been well described.ii

Mean (+/- SD) plasma chlortetracycline concentrations following oral administration of Aureomycin Granular (Alpharma) to 8 calves at 11mg/kg and 22mg/kg body wt/day divided over two daily feedings
The information for oxytetracycline is not as well referenced. An older study comparing OTC and CTC at 5 mg/lb in feed to cattle described serum concentrations of 0.1 to 0.21 μg/ml for CTC and 0.035 to 0.055 μg/ml for TC after 5 days.iii The CTC values would agree closely with the results reported above.
Ceftiofur
Pharmacodynamics are considered the same as for penicillins, time > MIC with durations above MIC not well established for veterinary pathogens.
Bovine Pharmacokinetic values for desfuroylceftiofur after ceftiofur sodium (1 mg/kg)iv
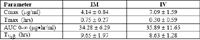
Only Cmax was significantly different between routes (P < 0.01)
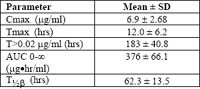
Bovine plasma concentrations of ceftiofur and metabolites after ceftiofur sodium administered intramuscularly or subcutaneously 1.0 mg/lb (2.2 mg/kg)

Comparison of ceftiofur sodium and ceftiofur hydrochloride in cattle: Fourteen head, 2.2 mg ceftiofur equivalents/kg, IM for both formulations

Excede label information for cattle: Ceftiofur crystalline free acid – 3 mg/lb BW in the posterior ear of cattle.
Susceptibility testing: Cephalosporin breakpoints adapted from CLSI M31-A3. Only ceftiofur and cefpodoxime (shaded) have CLSI validated breakpoints derived from veterinary data.

_____________________________________________________________________
i Sapadin AN, Fleischmajer R. Tetracyclines: Nonantibiotic properties and their clinical implications. J AM Acad Dermatol 54:258-265, 2010.
ii Reinbold JB, Coetzee JF, Gehring R, Havel JA, Hollis LC, Olson KC, Apley MD. Plasma pharmacokinetics of oral chlortetracycline in group fed, ruminating, Holstein steers in a feedlot setting. Journal of Veterinary Pharmacology and Therapeutics, 33:76-83, 2010.
iii American Cyanamid technical information bulletin No. 301, ANH-4150, April 1992.
iv Adapted from: Product Information Manual. Ceftiofur Sodium Sterile Powder For Use in Cattle. Pharmacia and Upjohn.