Guidelines for augmentation of circulating blood vome - Shock Fluid Therapy (Proceedings)
"Preload" parameters are those which address whether or not the heart is receiving a venous return sufficient to expect reasonable forward blood flow.
Recognition of an ineffective circulating volume
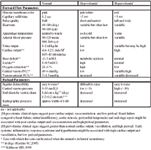
"Preload" parameters are those which address whether or not the heart is receiving a venous return sufficient to expect reasonable forward blood flow. "Forward flow" parameters are those which address the issues of stroke volume, cardiac output, arterial blood pressure and tissue perfusion. It is the forward flow parameters that are commonly and distinctly affected by aberrations in circulating blood volume and constitute the usual tip-off that there is a problem. The column titles "hypodynamic" are typical findings in hypovolemia, but may also be associated with any version of heart failure (congestive heart failure, mitral insufficiency, aortic stenosis, pericardial tamponade) or end-stage sepsis. The column titled "hyperdynamic" might represent the findings in hypervolemia, but are also typical of early systemic inflammatory response syndrome and hyperthermia.
The usual compensatory response to hypovolemia is an increase in heart rate and peripheral vasoconstriction (pale color, prolonged capillary refill time, cool appendages, and oliguria). Diminished pulse quality (height and width of the pulse pressure wave form) is the consequence of reduced stroke volumes secondary to the reduced venous return and tachycardia. Cardiac output would be proportionately reduced, but blood pressure is usually maintained until the compensatory mechanisms can no longer compensate. Metabolic markers of inadequate tissue oxygenation include an increasing base deficit, lactate, oxygen extraction, and venous-arterial PCO2 difference, and a decreasing central venous PO2. For all parameters, the greater the deviation from normal, the greater the underlying circulatory deficit. As for all cardiovascular or metabolic parameters, no one parameter defines a circulatory deficit; collect as many as possible in order to be the most secure in your assessment.
Poor forward flow parameters may be associated with hypovolemia but they may also be associated with poor heart function. In such cases it is possible to overload the heart (and cause pulmonary or systemic edema) prior to normalizing the forward flow parameters. When the forward flow parameters have not responded to a reasonable dose of fluids or if there is reason to suspect poor heart performance, fluids should be titrated to preload parameter end-points rather than forward flow parameter end-points (Table 8-1). When the preload parameters have been normalized and the forward flow parameters are still unacceptable, heart-specific therapy is indicated, such as sympathomimetic therapy if poor contractile function is suspected or pericardiocentesis if pericardial fluid is identified.
Goals of blood volume restoration therapy
It is always the ultimate goal of fluid therapy to restore the circulatory signs to normal. The immediate goal of fluid therapy, however, is acceptable (not necessarily normal) cardiovascular performance. The immediate goal of fluid therapy is to move the patient away from the "death line" so as to minimize chances that the animal might die as a consequence of the hypovolemia. The remaining fluid therapy to normalize the patient can be accomplished in a more leisurely (safe) manner. Previous attempts to immediately and completely normalize cardiovascular performance has resulted in edema and hemorrhagic complications. The goal of blood volume restoration therapy is to achieve what you want - restoration of acceptable cardiovascular performance, without causing what you don't want - edema or hemorrhage. Shock fluid therapy must be cautiously aggressive; large volumes are often required initially, but always with a eye on the preload parameters and the problems associated with the particular fluid being administered. If the preload parameters are high or a fluid-specific problem develops before acceptable forward flow parameters are achieved, a new plan must be developed.
Parameters
General principles of fluid therapy
While there is a bewildering variety of fluids from which to choose, they can be largely be grouped into a few categories with similar uses and indications.

General fluid categories
While circulating volume can be augmented by any fluid, there are vast differences between fluids with regard to their ability to accomplish this without harming the patient. This is partly determined by their redistribution characteristics after their administration and it is imperative to understand the physiology of fluid distribution across the vascular and cell membranes. Fluid choice is also partly determined by their solute content compared to normal blood; electrolytes, albumin, and hemoglobin concentrations cannot be allowed to get too high nor too low.
Isotonic crystalloids are commonly used to replace interstitial deficits in dehydrated patients and support circulating blood volume. Their use should be restricted in patients with interstitial edema. Artificial colloids are primarily used for blood volume and colloid osmotic pressure support and restoration. Plasma could be used for colloidal blood volume support, but this use is usually precluded by its expense. Plasma could also be used for albumin replacement, but in the face of ongoing albumin loss, this use is also limited by its expense. Plasma is, therefore, most often relegated to coagulation factor replacement. Hemoblobin products are primarily used for hemoglobin replacement in the anemic patient.
Fluid choices
There are many fluids (outlined below) and administration plans (outlined above) from which to choose for perioperative fluid therapy. For many patients it matters little which fluid or administration plan is selected. Clinicians should make selections based upon biologic appropriateness, cost, and technical aspects of administration. Since no one fluid or administration plan will be appropriate for all possible permutations of patient presentation, the clinician must also be ready to alter the standard approach to accommodate the specific needs of the patient.
Crystalloids
Crystalloids are fluids containing electrolytes (sodium (mw 23 Daltons); potassium (mw 39); chloride (mw 35.5); lactate (mw 89); acetate (mw 59); and gluconate (mw 195) and other small solutes such as glucose (mw 180) or mannitol (mw 182). These electrolytes and solutes are small in size compared to colloids and are freely permeable across the vascular endothelium. Crystalloids may be iso-osmotic, hypo-osmotic, or hyper-osmotic compared to normal extracellular fluid (which has a normal osmolality of about 300 mOsm/kg) depending upon their individual solute concentrations. More importantly, crystalloids my be isotonic, hypotonic, or hypertonic compared to normal extracellular fluid depending upon their sodium or effective osmole (mannitol but not glucose) concentrations.
Hypotonic crystalloids are probably poor choices for the primary fluid for perioperative fluid therapy because of the danger of water intoxication. Low-sodium solutions are primarily used for the replacement of water and some electrolytes necessary to maintain normal homeostasis. Five percent dextrose in water is primarily used as a delivery vehicle for drugs and as a source free water in the treatment of hypernatremia. Fifty percent dextrose in water is primarily used to supplement the glucose concentration of other administered fluids. For example, to increase the glucose concentration 2.5% in a volume of 300 mls of fluid simply multiply the volume of fluids (300) x the magnitude of glucose augmentation (0.025) to get the gms of glucose needed to add (7.5 gms; 15 mls of 50% dextrose).

Hypotonic solutions have a sodium concentration or osmolality less than plasma
Hypertonic solutions have a limited role in blood volume augmentation. Hypertonic saline (most commonly 7.5%) has been used in the initial management of severe hypovolemia. The recommended dosage is 4-6 ml/kg and is limited to this by virtue of the resultant acute hypernatremia. While the volume augmentation per volume of hypertonic saline administered is impressive, the clinical benefit is unimpressive and transient. There may be some use for hypertonic saline in ambulatory situations. The very concentrated hypertonic saline (23.4%) is primarily used to augment the sodium concentration of the primary fluid. Mannitol solutions are primarily used in the treatment of cerebral edema and as an osmotic diuretic.

Hypertonic solutions have a sodium concentration or osmolality greater than plasma
It is the isotonic crystalloids that are primarily used for perioperative fluid therapy. These crystalloids have a sodium concentration and osmolality that are near normal and these are largely restricted to the extracellular fluid compartment. In generally, it makes little difference which of these fluids is used; large volumes can usually be administered without adverse effects on normal electrolyte concentrations. One can see in Table 4, however, that there are some differences in chloride, potassium, and lactate/acetate/gluconate concentrations and these might be important in some circumstances. Saline tends to promote hyperchloremia, metabolic acidosis, and hypokalemia; Ringers solution tends to promote hyperchloremia and metabolic acidosis; and solutions with high concentrations of acetate and gluconate tend to promote metabolic alkalosis. Lactated Ringers solution, while having a near neutral effect on chloride, potassium, and acid base, also has the lowest sodium concentration. Lactated Ringers and Ringers solutions contain calcium but no magnesium; Plasmalyte 148® and Normosol R® contain magnesium but no calcium; saline contains neither. Sodium acetate has been associated with arteriolar vasodilation. Most normal patients can compensate for all of these effects, but none of the fluids are perfect and isotonic crystalloid fluid selection may be important in a select few patients.

Electrolyte concentrations of common crystalloids
When isotonic crystalloids are infused into the intravascular fluid compartment, they rapidly equilibrate with the interstitial fluid compartment in less than 30 minutes. This redistribution is the reason that such large volumes of isotonic crystalloids need to be administered for blood volume restoration (only about 20% of the infused volume remains within the intravascular fluid compartment). Interstitial crystalloid repletion is an advantage in dehydrated patients and a disadvantage in edematous patients.
If a patient is severely hypovolemic, it may require large volumes of crystalloids (20-40-60-80-100 ml/kg in 20 ml/kg aliquots associated with appropriate end-point monitoring) over short periods of time (a few minutes to several hours depending upon the degree of hypovolemia). Cats have a smaller blood volume than dogs and recommendations for fluid loading should be proportionately reduced (10-20-30-40-50-60 ml/kg in 10 ml/kg aliquots).
There are some general problems with any fluid that is administered rapidly or in large volumes. Heart failure patients are susceptible to fluid overload with any fluid; the heart cannot accommodate the enhanced venous return. Renal failure patients are susceptible to fluid overload if they cannot respond to it by increasing urinary loss.
Freshly lacerated/ruptured blood vessels (within the last hour) are subject to rebleeding if subjected to an aggressive fluid therapy plan with any fluid. An increase in vascular hydrostatic pressure cause rebleeding by disturbing the delicate platelet plug prior to clot stabilization. Pulmonary and cerebral contusions, lacerated livers and spleens, etc. are susceptible to this problem. It is possible to make a cerebral or pulmonary contusion worse or to convert a medically-treatable hemo-abdomen to a surgical hemo-abdomen with an aggressive fluid plan, but, unfortunatley, you will never know when this is about to happen, or has happened.
Fluid therapy in freshly traumatized patients should be cautiously aggressive; the goal is to be aggressive enough to move cardiovascular function up and away from the "death line" and yet conservative enough to avoid rebleeding.
There are also some crystalloid-specific problems that may occur when large volumes are administered rapidly. In addition to worsening a congestive heart failure, oliguric/anuric edema, and rebleeding, isotonic crystalloid fluids can cause edema by virtue of their substantial interstitial redistribution. Hemodilution of blood components that are not in the crystalloid fluid may also occur, characterized by anemia, hypoproteinemia, and diminished coagulation reserve. If any of these abnormalities become clinically significant during the administration of crystalloid fluids, red cells, colloids, or coagulation factors, respectively, must be added to the fluid therapy plan.
Saline solutions cause a transient metabolic acidosis while acetate/gluconate solutions can cause a transient alkalosis. Lactated Ringer's solution has a relatively neutral acid-base effect. The lactate in Lactated Ringer's will not enhance a lactic acidosis but may enhance the measured lactate if allowed to contaminate the blood sample. The iso-osmotic, polyionic fluids contain concentrations of potassium comparable to extracellular fluid. They cannot cause hyperkalemia and the potassium in them is not sufficient to replace a pre-existing potassium deficit. Acetated crystalloids decrease blood pressure when rapidly administered to some patients.
Artificial colloids
Colloid solutions contain large molecules (many thousand Daltons) that do not readily cross the normal vascular membrane. These solutions are largely retained within the vascular fluid compartment and therefore these fluids are more efficient blood volume expanders, and are less edemagenic, compared to crystalloids. Colloid solutions contain many different solute sizes. Molecular weights below about 50,000 Daltons are rapidly excreted in the urine. This causes a transient osmotic diuresis and can increase urine specific gravity (without a proportionate increase in urine osmolality). Larger molecules are slowly metabolized and tend to accumulate in the body with daily administration of the colloid. Dextrans not filtered by the glomerulus are metabolized by dextrinases in various tissues. Hetastarch and Pentastarch (Abbott® ) starches not excreted by the kidneys are metabolized by plasma and interstitial alpha-amylases, and plasma amylase may be elevated following their administration.
Commercial colloidal solutions are iso-osmotic (they are suspended in saline or another ECF-like crystalloid) and are hyperoncotic in the bottle. Artificial colloids are commonly administered to increase circulating blood volume and to support colloid osmotic pressure in hypoproteinemic patients. Loading dosages of colloid generally range from 5 to 30 ml/kg in 5 ml/kg aliquots (with appropriate monitoring of cardiovascular endpoints). For cats, the range is from 2.5 to 15 ml/kg in 2.5 ml/kg aliquots. Continuous rate infusions of colloids of 1 to 2 ml/kg/hr would be approximately equivalent to a crystalloid infusion of 5-10 ml/kg/hr, and could be used for intra-operative support of circulating blood volume. The same dosage rate has been used for support of colloid osmotic pressure in hypoproteinemic patients.
In addition to worsening of congestive heart failure, oliguric/anuric edema, rebleeding, and hemodilution, the artificial colloids produce a dose-related defect of primary hemostasis, which is somewhat greater than that due to hemodilution alone. Prolongation of aPTT is attributed to a reduction of VIII:C activity. Prolonged bleeding time and decreased platelet adhesiveness is attributed to inhibition of the vWf:ag. This effect on coagulation is more pronounced when colloids are administered in larger dosages or for longer periods of time and appear to be proportional to the molecular weight of the circulating macromolecules. It is not expected, however, that even large doses would induce bleeding in normal patients. However, some coagulopathic patients and some postsurgical patients may develop hemorrhagic problems. The coagulation effects of artificial colloids may be beneficial in the hypercoagulable phase of disseminated intravascular coagulation where an objective of therapy is to slow activated coagulation and platelet cascades.
Molecular sizes below 50,000 are rapidly excreted in the urine and this will cause a transient osmotic diuresis. The filtered colloidal molecules can be concentrated resulting in an increase in urine viscosity and specific gravity. In states of active tubular reabsorption (dehydration), the dextran molecules concentrate in the tubular lumen, increase filtrate viscosity and predispose to acute renal failure. Urine specific gravity is often used as a surrogate marker of urine concentration and when increased, it is usually taken as a sign of dehydration. Urine specific gravity must be interpreted with caution following or during artificial colloid administration; urine osmolality should be used instead as the marker of urinary concentration. Colloids may interfere with cross-matching procedures by causing red cell clumping. Allergic reactions are extremely rare, but are possible.
Plasma
Plasma might be administered for its albumin or coagulation factor content. Albumin is important to colloid osmotic pressure and because it is negatively charged is an important carrier of certain cations, drugs, hormones, metals, enzymes, chemicals, and toxins. Plasma, which has been separated from collected whole blood, could be administered to supplement albumin concentration and colloid osmotic pressure, but is expensive (compared to artificial colloids) and not very effective, particularly in the face of ongoing protein losses. Prepared plasma products, when they are diluted with anticoagulant, have an albumin concentration that is lower than it was in the donor. Large volumes would be necessary to affect albumin concentrations in the recipient.
The provision of coagulation factors is the most common reason to administer plasma. Whole blood and fresh plasma (within 8 hours of its collection) contains all of the coagulation factors and, depending on how it was separated, most of the platelets contained in the whole blood from which it is derived. A hard spin at 5000 rpm for 5 minutes places the platelets in the buffy coat; the plasma supernatant has no platelets. Platelet rich plasma is produced with a soft spin of about 2500 rpm for 2.5 minutes which does not separate the platelets from the plasma. Platelet rich plasma is produced by harvesting the buffy coat after a hard spin. Whole blood and plasma products intended for use for its platelets should be maintained at room temperature and gently rocked. Refrigeration or freezing destroys platelet activity. Fresh plasma and fresh frozen plasma is efficacious for the treatment of all coagulation disorders. Fresh frozen plasma (within 6 to 8 hours) preserves all of the labile (V, VII, and vonWillebrand's) and stabile (fibrinogen, II, VII, IX, X, XIII, and antithrombin) coagulation factors. Refrigerator storage is associated with a significant loss of factor VIII and vonWillebrand's activity by 24 hours and of factors V and XI by one week. Cryoprecipitate provides a concentrated source of factor VIII, XIII, von Willebrand's, fibrinogen, and fibronectin. Refrigerator stored plasma, plasma frozen after 8 hours post-collection, and fresh frozen plasma beyond one year still contains the stabile factors and is effective for the treatment of warfarin-related rodenticides.
In addition to worsening of congestive heart failure, rebleeding, and dilutional anemia, plasma administration is relatively expensive. Homologous albumin infusions may occasionally be associated with a transfusion reaction. Twenty-five percent human albumin has been administered to dogs and very effectively augments plasma protein concentration and colloid osmotic pressure. First time infusions, however, have been associated with acute and delayed transfusion reactions.
Hemoglobin
In humans, the trigger for a hemoglobin transfusion has traditionally been a hemoglobin concentration of 10 g/dL (a packed cell volume [PCV] of 30%), however recent studies suggest that a more relaxed trigger of 7 g/dL (PCV = 21%) might represent a better benefit:risk ratio. In veterinary medicine, a packed cell volume of 20% has been a common trigger for blood transfusion, however, given the complexities of cardiac output and oxygen-extraction compensatory mechanisms, it is not possible to precisely define a minimum hemoglobin concentration. Anesthetic agents decrease myocardial contractility and cardiac output and it would be predicted that anesthetized patients require a relatively higher hemoglobin concentration (7-8g/dl). Metabolic markers of poor tissue oxygenation, such as a low PvO2 or a metabolic (lactic) acidosis, may help guide the need for hemoglobin transfusions.
Whole blood may need to be administered in volumes of 10 to 30 ml/kg, depending on the magnitude of anemia and hypovolemia (cats: 5 to 15 ml/kg). These volumes should be halved if packed red blood cell products are used. The rate of administration depends upon the magnitude of the hypovolemia. The amount of blood to administer can also be calculated: (desired PCV - current PCV) x body weight (kg) x 2 ml whole blood (assumes a PCV of about 40%) (or 1 ml packed red blood cells [assumes a PCV of about 80%]).
Although many canine erythrocyte antigens have been identified, only 6 (DEA 1.1, 1.2, 3, 4, 5, and 7) are commonly tested. Dog erythrocyte antigen 1.1 and 1.2 are the most antigenic and are present in approximately 62% of the canine population. Fortunately naturally-occurring isoantibodies to DEA 1.1 and 1.2 do not exist and, in this regard, first-time transfusions are relatively safe. Donors and recipients should be DEA 1.1 and 1.2 typed to assure that recipients receive type-similar blood. DEA 1.1 and 1.2 negative recipients should not receive type-positive blood because it would prime them from a second-time transfusion reaction. The other four canine blood groups are weak antigens as are any naturally-occuring isoantibodies and transfusion reactions are mild if they occur at all. In vitro cross matching would help sort out potentially incompatible transfusion from any cause and may be important for sequential transfusions and in immune-mediated hemolytic anemia.
There are primarily two commonly identified red cell antigens in the domestic cat — A and B (a third type, AB, is rare). The domestic "mongrel" cats, Siamese, Burmese, and Russian Blue breeds are typically Type A. Type B blood types occur in up to 10% of Maine Coon cats, up to 20% in Abyssinian, Birman, Persian, Somali, Spinx, and Scottish Fold cats, and up to 45% in British Shorthair, and Cornish and British Rex cats. Type A cats have low titers of naturally occurring anti-B antibodies. Type B cats have high titers of strong, naturally occurring anti-A antibodies. Matched transfusions were associated with a mean survival time of labeled red cells of 29 to 39 days. Transfusion to Type B blood into Type A cats was associated with a mean red blood cell survival time of 2 days and minor transfusion reactions, while transfusion of Type A blood into Type B cats was associated with a mean red blood cell survival time of 1 hour and marked transfusion reactions. Feline blood transfusions should by type-matched, either by blood typing of both donor and recipient or by in vitro cross-matching.
If not administered through a filter, infused blood may cause pulmonary emboli from small clots or cell aggregations. These transfusion-related emboli can cause an acute lung injury syndrome indistinguishable from other causes of respiratory distress syndrome. Hypothermia may occur if a large volume of cold blood is administered rapidly. Hypocalcemia may occur if large quantities of citrate are administered; patient heparinization may occur if large quantities of heparin are administered; and acidosis may occur if large quantities of acid citrate anticoagulants are administered or if the blood has been stored longer than 1 week.
All blood and plasma transfusions should be monitored for immunologic transfusion reactions whether or not typing/cross matching has been done. When clinical conditions permit, blood should be administered slowly at first; if there is a transfusion reaction, the transfusion can be stopped before excessive amounts of antigen have been administered. Immunologic transfusion reactions may be manifested by fever, hemoglobinemia or hemoglobinuria, restlessness, hypotension, acute collapse, wheezing, dyspnea, or urticaria. If any of these develop during a blood transfusion, it should be stopped immediately. Antihistamines or corticosteroids are not routinely administered unless a transfusion reaction has been observed.