Endoscopic examination of mares (Proceedings)
Endoscopic examination of the mare's uterus is primarily used as tool to aid in the diagnosis of unusual conditions, such as intractable infections, tumors, abscesses, foreign bodies, adhesions, and congenital abnormalities.
Indications for endoscopic examination of the mare
Endoscopic examination of the mare's uterus is primarily used as tool to aid in the diagnosis of unusual conditions, such as intractable infections, tumors, abscesses, foreign bodies, adhesions, and congenital abnormalities. Endoscopy is also used to perform assisted reproductive procedures such as hysteroscopic insemination. In certain cases it is used as an adjunct procedure during a breeding soundness examination, as a follow-up after a reproductive surgery, dystocia, or to perform a targeted uterine biospy or foreign body retrieval. In post partum mares it may used to assist with the diagnosis of uterine ruptures. One of the most common usages is to investigate unexplained fluid accumulations in mares, and to monitor the progress to therapy.
Anatomic features viewed using endoscopy
A clinical examination of a mare should begin with observations regarding the mare's perineal integirty. An endoscope may be used to view the interior of the vagina, the cervix and the uterus. In the vagina the, vestibulovaginal fold, hymen in maiden mares, an external cervical os may be viewed. The interior of the cervical canal may be examined and the uterine: body, bifurcation, horn, uterotubal papilla and ostium. Structures of interest in the vagina include the urethral tubercule, and venous varicosities that often are located dorsally in the region of the vestibulovaginal fold, and in the uterus, endometrial cysts, and endometrial lymphatic cysts. Endoscopy has been successfully used to identify and remove foreign bodies such as broken culture swabs. It is and is used to determine the status and health of the endometrium, to sample discharges, perform a biopsy of a specific area, or to evaluate the nature and condition of tumours or hematomas, explore anomalies such as adhesions, and identify congenital abnormalities.
Hysteroscopy - hysteroscopic anatomy
The uterus in the mare is Y shaped with a very short body that bifurcates and leads to dependently located uterine horns that then rise toward the ovary. Longitudinal folds of endometrial mucosa that form ridges which run from the body into the uterine horns, these folds provide the "spoke wheel" appearance on ultrasound examination when the uterus tissue is edematous. The dimensions of the uterine horns are 15 - 20 cm long and 4 - 7.5 cm in wide, smaller than most people realize. The longitudinal folds lead to the end of the uterine horn where the uterotubal papilla is located dorsally an eccentrically. Endoscopic figures are seen in panels 1 – 3.
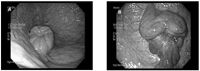
Figure 1A: Normal estrus cervix, B: Inflammed and edematous external cervical os
The endoscopic procedure
The mare is prepared as if for AI by emptying the rectum of manure, wrapping the tail, and cleansing the perineum. Generally mares are sedated using butorphanol and an alpha agonist such as xylazine or detomidine, to minimize movement and any discomfort. Tractable mares may not need to be sedated, however the endoscopic equipment is very expensive and should be carefully monitored. The mare is positioned in a stocks following sedation so her airway is unobstructed. The endoscopic equipment is gas or chemically sterilized and the disinfectant removed using copious amounts of alcohol, of sterile water or saline. One examiner wearing a rectal sleeve and sterile surgical glove, passes the equipment through the vagina, cervix and into different uterine locations (body and both horns), and an operator directs the tip of the endoscope when required. A one meter gastroscope, or videoendoscope is recommended. The cervical canal is gently dilated by the examiner's finger and the endoscope is passed into the uterine body. The cervix is held closed around the endoscope and the uterus is insufflated for around 20 seconds using air. Following the intial insufflation the air pressure is maintained using intermittent or low air flow during the hysteroscopic examination to keep the mare comfortable. Too much insufflation pressure will make the mare move around and gas may be released through the cervix. The contents of the uterus may be fully visualized using pressures of 17.8 -30 mmHg and may be regulated. It is important to remember the diameter of the scope may occupy about half the lumen of the uterus, therefore very gentle intentional movements should be performed when is good insufflation and visualization is present. The uterine body is examined and then the endoscope is passed cranioventrally to the bifurcation of the uterine horns. The endometrial folds are followed to the tip of the uterine horn. The examiner, using their hand in the mare's vagina and another on the endoscope, feeds and directs the endoscope by watching the progress on the videoscreen. Using direct visualization samplesof uterine discharge, fluid aspirates, or guided endometrial biopsies may be collected by passing tools such as catheters or endoscopic tools through the biopsy port of the endoscope. A Piling endometrial biopsy instrument is passed through the cervix alongside the endoscope until it comes into view for targeted biopsies. After a biopsy bleeding is common therefore the biopsy is usually taken last. When the procedure is complete and the entire contents of the uterus have been viewed, the air flow is shut off and the biopsy port opened, the air released, and a rectal massage is used to encourage deflation of the uterus. The equipment should be immediately disinfected.
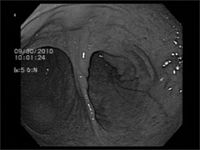
Figure 3: Hysteroscopic view of the bifurcation of the uterine horns.
Post partum uterus
The post partum uterus is large and may contain lochia or other inflammatory debris. Evaluation of the post partum uterus using hysteroscopy is difficult to perform and insufflation more difficult to achieve due to the large volume, large cervix, and heavy endometrial folds. The uterus of the mare may be injured during foaling and full-thickness lacerations of the dorsal portion of the uterine horn have been reported, sometimes only bruising is noted on the endoscopic examination. In some mares a portion of the placenta may also be retained in the uterine horns, particularly the tip of the non-pregnant horn.These mares presented as a depressed post-partum mares. Ultrasound and endoscopy may be applied to confirm the diagnosis in these cases.
Appearance of the endometrium
While performing the procedure the examiner should focus on colour and texture of the vagina initially and then the cervix and finally the uterus. The vagina of the mare is pink and the vulva loosens when the mare is in estrus.The cervis is dynamic and during the breeding season varies in position and degree of relaxation due to steroid hormone influences. Estrogen causes the cervix to relax, progesterone tightens the cervix. The cervical canal has a smooth pink interior. Note the presence of discharge in the vagina, and in the uterus foreign matter, endometrial cysts, endometrial lymphatic cysts, masses, presence of adhesions, plaques, discoloured regions, lymph stasis or abnormal septae. The endometrium of the mare is pale pink and in estrus and is covered with a glistening mucus, the endometrial folds will be edematous and it should have a homogenous texture. The diseased endometrium may have ulcers, plaques, discoloured patches, discharge, and visible ymphstasis evident. The endometrium of subfertile mares may have a pronounced pattern that corresponds to lymphstasis and endometrial gland necrosis. Pyometra is a persistent condition where the uterus fills with pus, typically in association with a cervical injury such as adhesions, that mechanically interfere with cervical dilation or patency. The endometrium may be diseased,and may be incapable of secreting prostaglandin, resulting in prolonged luteal phases. Transluminal uterine adhesion may be treated using laser surgery.Transendoscopic electrosurgery and LASER has been used to reduce adhesions.
Endometrial glandular and lymphatic cysts
In the uterus there are 2 types of cystic structures endometrial glandular cysts and endometrial lymphatic cycsts. The endometrial glandular cysts are less than a few mm in diameter, while the endometrial lymphtic cysts vary from small to large and may be present as solitary cysts, or clusters. Lymphatic cysts may be confused with embryos on ultrasound examinations because they may be round The cysts vary in significance but generally do not interfere with fertility and grow very slowly over time. Therefore clinically it is important to note the location and map the size of endometrial cysts in the uterus. The endometrial cystic structures do not develop heart beats and grow slowly, embryos develop heart beats and grow quickly. When there is no previous examination history, a mare should not be diagnosed as pregnant until an embryo with a heart beat is identified, and terminology like a structure "compatible with an embryonic vesicle" is recorded.
Hysteroscopic insemination
Hysteroscopic insemination and deep horn insemination are techniques for low dose insemination. A reduced number and minute insemination volume are used in this technique. The side where the dominant follicle is located is determined. The endoscope is directed up the uterine horn ipsilateral to the dominant follicle. A small catheter is passed through the biopsy channel of the endoscope and advanced so it touches the uterotubal papilla. The inseminate is then injected onto the surface of the papilla. This technique has been successful in achieving pregnancies with low numbers of fresh, frozen thawed sperm.
Congenital abnormalities
Remants of paromesonephric ducts (persistent Müllerian ducts) are Congenital and are frequently investigated using hysteroscopy. Paramesonephric duct remnants include a longitudinal septum like division of the vagina, cervical aplasia, double cervix, uterus unicornis, and uterus bicollis. Ectopic ureters have also been reported in the mare.
Persistent endometrial cups
Persistent endometrial cups are found in some bred or post foaling mares that fail tocycle, bur are a rare finding. Cup tissue may be visualized using endoscopy, and is associated with high hormonal levels of eCG, and typically these mares undergo follicular luteinzation rather than ovulation. Persistent anovulatory follicles may be a feature of these mares.
Tumours of the uterus
Solid masses in the uterus may be tumours, or intramural hematoma formation. Intramural hematomas form as a foaling injury, and slowly resolve over time. The most common uterine tumor is comprise of smooth muscle and is called a leiomyoma. Leiomyomas are usually first detected using transrectal ultrasound examination. They are slow growing, range in size from 2 – 5cm, and may be pedunculated. As the tumour enlarges it may obstruct the uterine lumen, become necrotic on the surface as it outgrows its blood supply, and subsequently interferes with fertility. Uterine adenocarcinomas have also been reported and result in significant tissue damage, and frequently uterine discharge. Tumors are removed surgically. A mare must have 70% of her uterus remaining following surgical removal of the tumor for successful pregnancy. Lymphosarcoma and melanoma may also metastasize to the uterus.
Foreign bodies
Most foreign bodies in the mare's uterus are from manufacturing defects in swabs, or rough handling of equipemnt. The broken culture swabs may be retrieved by manually by dilating the cervix and retrieving the missing piece, or by using uterine lavage to flush out the pieces. In cases where this is not successful implements such as endoscopic biopsy tools, or a biopsy forcep may be used to grab or dislodge a foreign body that is embedded or trapped in the endometrium. The foreign body is then withdrawn as the endoscope is withdrawn from the uterus.
Fetal maceration or mummification
Occasionally a fetus dies and the mummified or macerated bones are retained in the mare's uterus. The bones may be adherent to the endometrium and require manipulations such as bringing the mare into heat and using manual cervical dilation or use of tools through the endoscope's biopsy port to dislodge them.
References
Bracher, V., S. Mathias, and W.R. Allen, Videoendoscopic evaluation of the mare's uterus: II. Findings in subfertile mares. Equine Veterinary Journal, 1992. 24(4): p. 279-284.
Wilson, G.L., Equine hysteroscopy: a window to the internal reproductive tract. Veterinary Medicine & Small Animal Clinician, 1983. 78(9): p. 1455-1459, 1462-1466.
Ball, B.A., Hysteroscopic and low-dose insemination techniques in the mare. Ippologia, 2006. 17(3): p. 25-30.
Morris, L.H.A. and W.R. Allen. An overview of low dose insemination in the mare. in 6th Annual Meeting of the European Society for Domestic Animal Reproduction, University of Parma, Italy, 12-14 September 2002. p.206-210.
Lindsey, A.C., Schenk J., Graham J., Bruemmer, J., Squires E.L. Hysteroscopic insemination of low numbers of flow sorted fresh and frozen/thawed stallion spermatozoa. Equine Veterinary Journal, 2002. 34(2): p. 121-127.
Wilson, G.L., Equine hysteroscopy: normal intrauterine anatomy. Veterinary Medicine and Small Animal Clinician, 1984. 79(11): p. 1388-1393.
Card, C.E., Eaton, S.E.,Ghasemi, F. How to perform a hysteroscopically assisted endometrial biopsy and foreign body retrieval in mares. Proceedings AAEP 2010. 66:328-330.Baltimore, MD.
Bracher, V. and W.R. Allen, Videoendoscopic evaluation of the mare's uterus: 1. Findings in normal fertile mares. Equine Veterinary Journal, 1992. 24(4): p. 274-278.
Leidl, W. and U. Schallenberger-Pottiez, Hysteroscopy in the mare. Veterinary Medical Review, 1976(2): p. 203-217.
Berezowski, C., Diagnosis of a uterine leiomyoma using hysteroscopy and a partial ovariohysterectomy in a mare. Canadian Veterinary Journal, 2002. 43(12): p. 968-970.
Menzies-Gow, N., Diagnostic endoscopy of the urinary tract of the horse. In Practice, 2007. 29(4): p. 208-213.
Wilson, G.L., Hysteroscopic examination of mares. Veterinary Medicine & Small Animal Clinician, 1983. 78(4): p. 568-578.
Bartmann, C.P. and V. Schiemann, Establishment of a standardized intrauterine distension pressure for the hysteroscopy in the horse. Deutsche Tierarztliche Wochenschrift, 2003. 110(2): p. 43-48.
Hecker, C. and R. Hospes, Endometrial biopsy in the mare - "blind" sampling of tissue or under hysteroscopic control? Pferdeheilkunde, 2006. 22(2): p. 140-144.
Schiemann, V., Bartmann, C. P., Kirpal, G., Reiswitz, A. v, Schoon, H. A., Klug, E., Diagnostic hysteroscopy in the mare - uterine contamination and endometrial reaction. in 3rd International Conference on Equine Reproductive Medicine, Leipzig, Germany, 27-28 October 2001. p. 557-564.
Ferrer, M.S., Lyle, S. K., Eilts, B. E., Godke, R. A., Paccamonti, D. L.Post-mating endometritis after low dose hysteroscopic insemination in the mare. Theriogenology, 2005. 64(3): p. 784-784.
Bartmann, C.P., I. Brickwedel, and E. Klug, Trans-endoscopic high frequency electrosurgery for minimal invasive treatment of intrauterine adhesions in mares. Tierarztliche Praxis. Ausgabe G, Grosstiere/Nutztiere, 2000. 28(4): p. 233-239.
Schiemann, V. and C.P. Bartmann, Transendoscopic synechiolysis of extensive intrauterine adhesions by repeated operative hysteroscopy in a mare - a case report. Pferdeheilkunde, 2003. 19(6): p. 661-665.
Kaspar, B., Kahn, W., Laging C., Leidl, W., Endometrial cysts in mares. I. Post mortem studies, occurrence and morphology. Tierarztliche Praxis, 1987. 15(2): p. 161-166.
Leidl, W., B. Kaspar, and W. Kahn, Endometrial cysts in the mare. 2. Clinical examinations: frequency and importance. Tierarztliche Praxis, 1987. 15(3): p. 281-289.
Manning, S.T., Bowman, P.A, Fraser L.M., Card, C.E. Development of Hysteroscopic Insemination of the Uterine Tube in the Mare in Proceedings of the American Association of Equine Practitioners. 1998. Baltimore, MD.p. 84-85.
Vasquez JJ, Medina, V., Liu I.K.M., Ball B.A., Scott M.A., Non-surgical uterotubal insemination in the mare. in American Association of Equine Practitioners. 1998. Baltimore, MD.p. 68-69.
Lindsey, A.C., Morris, L. H. A., Allen, W. R., Schenk, J. L., Squires, E. L., Bruemmer, J. E., Hysteroscopic insemination of mares with low numbers of nonsorted or flow sorted spermatozoa. Equine Veterinary Journal, 2002. 34(2): p. 128-132.
Hollerrieder, J. and J. Toth, Minimal invasive surgery of a bleeding uterine tumor in a mare using a wire cerclage. Tierarztliche Praxis. Ausgabe G, Grosstiere/Nutztiere, 2002. 30(6): p. 404-407.
Janicek, J.C., D.H. Rodgerson, and B.L. Boone, Use of a hand-assisted laparoscopic technique for removal of a uterine leiomyoma in a standing mare. Journal of the American Veterinary Medical Association, 2004. 225(6): p. 911-914.
Neufeld, J.L., Lymphosarcoma in a mare and review of cases at the Ontario Veterinary College. Canadian Veterinary Journal, 1973. 14(7): p. 149-153.