Choosing antimicrobials (Proceedings)
Patients in the ICU can require antimicrobial therapy for numerous reasons.
Patients in the ICU can require antimicrobial therapy for numerous reasons. Patients may be admitted to the ICU with an ongoing infection or they may develop an infection while in the ICU, such infections are referred to as hospital-acquired infections. Early appropriate antimicrobial treatment is essential since incorrect antimicrobial choices are associated with increased mortality. While it might seem prudent to put all ICU patients on antibiotics, this will ultimately lead to an increase in antimicrobial resistance.
Antimicrobial therapy should be tailored to maximize benefits while minimizing risks. Ideally, therapy should be based upon results of an antimicrobial culture and sensitivity report. In cases where such a report is not available rational empiric therapy requires knowledge of the likely infecting organism(s) as well as the spectrum of activity of potential antimicrobial therapy. Only by assimilation of this knowledge can the clinician consistently make rational antimicrobial choices. It is the responsibility of the clinician to collect appropriate samples for culture and sensitivity in all cases where feasible. Random changes in antimicrobial therapy not guided by antimicrobial cultures can result in inadequate antimicrobial coverage as well as leading to the development of antimicrobial resistance
- Empirical antimicrobial therapy – The use of an antimicrobial in the absence of an etiologic diagnosis.
o Life-threatening infections
o Failure to culture an organism
o Economic considerations
- Selection of an empirical agent
o Knowledge of likely pathogens
o Knowledge of drug pharmacokinetics
• Bioavailability
• Concentration at infection site
o Potential toxicities
o Cost of treatment
o Regulatory concerns
Susceptibility testing
Some organisms have highly predictable antimicrobial susceptibility patterns and as a result microbiology laboratories do not routinely perform susceptibility testing but rather provide a list of antimicrobials that are predictably efficacious (coagulase-negative Staph., Rickettsia). Susceptibility testing is generally performed by either the disk diffusion or dilution methods. The distinction between antimicrobial sensitivity and resistance is based upon the determination of a minimum inhibitory concentration (MIC). The MIC is the minimum antimicrobial concentration required to inhibit growth. The MIC is established by the National Committee for Clinical Laboratory Standards based upon available pharmacokinetic and microbial data. Intermediate sensitivities represent organisms resistant to typical therapeutic concentrations that may be sensitive to maximally achievable concentrations (i.e. urine concentration).
Antimicrobial dosing regimens
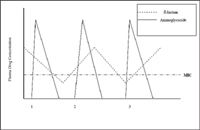
Typically, antimicrobial dosing regimens are designed to maintain tissue and plasma drug concentrations above the target organisms MIC. This approach usually necessitates repeated dosing throughout the course of a day. Recently, the existence of a post-antibiotic effect (PAE) has been recognized. The PAE refers to the existence of residual antimicrobial effects despite serum and tissue levels below the MIC. The two drug classes commonly used in veterinary medicine that exhibit a PAE are the fluoroquinolones and the aminoglycosides.
Antimicrobial resistance
The growing problem of antimicrobial resistance has been identified as one of the greatest threats to human health in the 21st century by the Centers for Disease Control and Prevention. As veterinarians we play a key role in limiting the development of this problem through our use of antimicrobial drugs. The development of antimicrobial resistance can occur through either spontaneous chromosomal mutations or the transfer of R plasmids between microorganisms. Plasmid transfer represents the major source of antimicrobial resistance. R plasmid transfer is uncommon in most Gram positive organisms except for Staph sp. and Enterococcus. R plasmid transfer occurs much more frequently between Gram negative organisms. A single R plasmid may code for the resistance of up to 10 antimicrobials.
Limiting antimicrobial use and using them appropriately when they are indicated are the two most important strategies to limit the development of resistance. Antimicrobial therapy should be prescribed for the minimum duration needed to result in a clinical cure. Therapy beyond this point is not of any benefit and only serves to heighten the problem or resistance. Subtherapeutic dosing levels also result in the promotion of resistance (such as antimicrobial use as a growth promoter in food animals). The narrowest spectrum drug available to treat the infection should be chosen and therapeutic choices should be refined once sensitivity results are available.
Prophylactic antimicrobial therapy
Prophylactic antibiotics are only indicated in very specific instances. These include orthopedic procedures, contaminated abdominal procedures, and potentially, neutropenic oncology patients. Surgical prophylaxis should be timed so as to maximize drug tissue concentrations at the time of the first incision. Generally, this means the antibiotic should be given 60 – 15 minutes before the actual start of surgery (not anesthesia induction). The antimicrobial should be redosed as needed to maintain high tissue concentrations (generally every 2 hours). There is no advantage to continuing therapy beyond 24 hours. Potential disadvantages of prophylactic therapy include toxicity, drug resistance, cost, and antimicrobial residues in food animals.
Reasons for therapeutic failure
There can be numerous causes for therapeutic failure. The most common is that the underlying disease is not bacterial in origin. When initial therapy fails the clinician should reexamine the diagnosis and confirm a bacterial origin instead of simply changing antibiotics. Other reasons for failure include drug interactions, walled off infection preventing antimicrobial penetration, inadequate host immune response, the presence of drug resistance, and inadequate time for response. It can take up to 72 hours to see clinical improvement from antimicrobial therapy.
Patients with hospital-acquired infection are typically identified by the development of fever, tachypnea or dyspnea, or purulent discharge from an incision or wound. Since such infections frequently develop as a result of antimicrobial resistant infections, hospital-acquired infections require treatment with antibiotics that are known to be effective against the pathogens frequently found in an individual ICU. In the Tufts University School of Veterinary Medicine's ICU hospital-acquired infections are typically resistant to most antibiotic choices with the exception of imipenem and amikacin. As a result, patients with hospital-acquired infections are started on one of these antibiotics pending culture and sensitivity results.
Antimicrobial treatment of ICU patients requires knowledge of likely pathogens, spectrum of activity of available antibiotic choices, and the resistance patterns typically seen in hospital-acquired infections. Only by integrating all of this knowledge can appropriate antimicrobial choices be made.