Cardiopulmonary resuscitation (Proceedings)
Veterinary and human studies cite a 30 to 60% rate of return of a spontaneous beating heart.
Success rate
Veterinary and human studies cite a 30 to 60% rate of return of a spontaneous beating heart10,30,49,90 . Unfortunately, the disease that induced the cardiac arrest in the first place is still present, and is now magnified by the ravages of the cardiac arrest and resuscitation experience. Veterinary and human studies cite much lower "go-home" statistics: 2 to 24% 10,30,42,49,76,90 . In human studies, many of those that are discharged have significant and persistent neurological deficits. A low percentage, however, is not a zero percent and it makes a big difference for those few individuals in which the resuscitation is ultimately successful.
A human patient has the moral and legal right to choose or decline any therapy, including cardiopulmonary resuscitation, irrespective of the presence of terminal disease or the mutual consent of family members or the physician1 . In veterinary medicine, the owner of the animal, as the legal surrogate decision maker for that patient, has the same rights and obligations. Informed consent means that the individuals making the decision understand the basic information about their animal's condition and the prognosis, the nature of the proposed intervention (the resuscitation), the risks and benefits, and the consequences.
Resuscitation is not appropriate for every patient that has a cardiac arrest. It was always only intended for patients that might have a treatable underlying disease where there is likelihood of return to an acceptable quality of life. Whether or not to resuscitate an arrested animal is a decision that must be made on the merits of each individual case and in concert with the owner's wishes. These decisions must be made in advance whenever possible.
Equally important is the decision as to when to stop a resuscitation. The resuscitation technique should continue for as long as there was any chance for obtaining a spontaneously heart beat. Dogs arrested (hypoxic) and untreated for 10, 15 and 20 minutes had a 100%, 50%, and 29%, respectively, return of spontaneous circulation and 71%, 33% and 0%, respectively, one-hour survival31 . Dogs subjected to closed-chest compression for 15, 20 and 25 minutes before open-chest CPR had a 75%, 38%, and 0% resuscitation rate88 . In human clinical studies, no survivals were recorded in patients requiring resuscitation for longer than 30 minutes 10,39,45,70,90 . The average length of time to successful resuscitation in dogs was 17 minutes (95% confidence interval 7 to 27 minutes) and in cats was 21 minutes (95% confidence interval 8 to 34 minutes) 49 . While it is very presumptive to designate a timed stopping point (such decision should be made on the merits of the case in so far as they can be determined), it would seem that 30 minutes is about as long as one could reasonably expect to be able to restore a spontaneous heart beat and expect to be able to discharge a reasonably neurologically intact patient from the hospital.
Airway and breathing
The airway should be secured by endotracheal intubation, and positive pressure ventilation with 100% oxygen instituted as a first priority. One ventilation should be delivered approximately every 3 to 5 chest compressions and should be delivered between chest compressions without pausing the compression procedure. If the resuscitation is being conducted by one person, two ventilations should be delivered approximately every 15 chest compressions. The ventilation goal is moderate hyperventilation to make sure that hypercapnia is avoided.
There is some discussion in human medicine that defibrillation, rather than airway and breathing should be the first step in the CPR algorithm. The is based on two facts. First, ventricular fibrillation is a very bad thing. Ventricular fibrillation is exercising muscle; metabolic rate and oxygen demands are high, vascular resistance is high, and arterial blood pressure, perfusion pressure and oxygen delivery is low. Survival decreases 7-10% per minute and is virtually zero after 12 minutes43,57,67,71,111 . Second, in adult humans, 85% of all cardiac arrests that occur in middle-aged to older adults and are due to ventricular tachyarrhythmias and ventricular fibrillation secondary to myocardial infarction or cardiomyopathy1,9 . Prior to 5 minutes, defibrillation emerges as the highest priority; after 5 minutes, re-oxygenation (includes PPV with oxygen, artificial circulation, and epinephrine) may be the highest priority, even in the fibrillating patient29,71 . In children and young adults1 , and in veterinary patients, cardiac arrest is usually asystolic, due to hypoxemia secondary to pulmonary or neurologic impairment, or to myocardial hypoxia secondary to cardiovascular or metabolic impairment. Airway and breathing would seem to be the most important immediate treatment for veterinary patients, most of the time.
In humans, for reasons of fear of infectious disease, there is reluctance to perform mouth-to-mouth resuscitation. This prompted research into the effects of chest compression without ventilation, and it appears that such a procedure is better than nothing at all1 . Some studies suggest that ventilation is not absolutely necessary during the first 6 to 12 minutes of resuscitation26,74,100 . We are not proposing a no ventilation CPR algorithm, but if your situation precludes airway access and ventilation at the moment, there is some evidence to suggest that, until an endotracheal tube arrives, that chest compression alone is better than nothing at all and is not a waste of energy.
Circulation
External chest compression
External chest compression should be accomplished by applying pressure directly over the heart with a force that is appropriate for the size of the patient at a rate of 80 to 120 times per minute. This may be done in lateral recumbency, applying pressure to the lateral thoracic wall, or in dorsal recumbency, applying pressure to the sternum. The compression should be held for a brief period of time to maximize the elimination of blood from the heart and the chest. Time must be allowed between compressions for adequate diastolic filling of the ventricles. The technique is never interrupted.
The effectiveness of the compression technique must be evaluated frequently (continuously if possible). The palpation of a peripheral pulse in the femoral, dorsal metatarsal, or lingual artery is evidence of a generated stroke volume. It is not, however, a guarantee that cardiac output, arterial blood pressure, or tissue perfusion is adequate. An improvement in the color of the mucous membranes of the mouth also provides evidence of some tissue perfusion. A doppler flow probe, placed on the eyeball, has been used to assess retinal blood flow during CPR. End-tidal carbon dioxide measurements can also be used to assess tissue blood flow. If there is no blood flow, the end-tidal CO2 will be zero (normally it is slightly below PaCO2). End-tidal CO2 increases in association with improved tissue perfusion40 . End-tidal CO2 measurements above about 15 mmHg were reported to be associated with higher survival rates21,102 .
The specific technique that will net an effective forward blood flow varies markedly from patient to patient. If the initial technique does not generate any evidence of forward blood flow, an alternate technique should be used. The compression force could be increased or decreased, the rate could be increased or decreased, the duration of systole could be increased, the position of the animal could be changed, the position of the hands or compressor with respect to the patient could be changed, or the compressor could be changed.
Abdominal counter-pressure helps splint the abdomen and decreases the posterior displacement of the diaphragm when the chest is compressed. This prevents the dissipation of the intra-thoracic pressure induced by chest compression out through the abdomen. The technique enhances the generalized increase in intrathoracic pressure and improves cardiac output and cerebral blood flow. Our experience has been than abdominal counter pressure can increase diastolic arterial blood pressure during external chest compression by as much as 20 mm Hg. Abdominal counter pressure can be applied by the hands of an assistant, a sandbag, or a large book (like this one!). A snug abdominal wrap would also be effective in this regard but is very difficult to apply in an emergency situation.
Antishock trousers have been reported to improve systemic blood pressure and vital organ perfusion by returning a small amount of blood from the peripheral pool to the central circulation and by preventing the runoff of central blood volume into the periphery. In veterinary medicine, antishock trousers can be facsimilied by wrapping the hind legs and caudal abdomen with elastic bandaging material. Starting from the toes, wrap both hind legs and the tail; wrap up to the caudal abdomen. Such a wrap is difficult to apply to dogs because the triangular shape of the thighs which causes the wrap to slide down on the leg. Care should be taken with the abdominal portion of the wrap not to wrap too far forward on the abdomen (to make a Greyhound out of a Beagle) because it will cause anterior displacement of abdominal contents. Thoracic compression is then more likely to fracture the liver resulting in abdominal hemorrhage. A single abdominal tourniquet may be just as efficacious as shock trousers. A rope, belt, or gauze can be tightly placed around the lower abdomen, just anterior to the pelvis; tight enough to compress the descending aorta. Wraps and tourniquets can be removed 10 to 20 minutes after restarting the heart; after hemodynamics have had a chance to stabilize. Wraps and tourniquets should be removed slowly; rapid exposure of a precariously balanced cardiovascular system to the hypoxic vasodilated tissues caudal to the tourniquet or under the wrap may result in excessive hypotension.
Intermittent abdominal compression, alternating with external chest compression, improves venous return to the chest and has been reported to improve arterial blood pressure, cerebral and myocardial perfusion, and survival13,80,102 . A second person compresses the abdomen between each chest compression. This increases the abdomen-to-chest pressure gradient and enhances the volume of intrathoracic blood to be expelled during the next chest compression.
Simultaneous ventilation and external thoracic compression have been shown to enhance the generalized increase in intrathoracic pressure and to improve cerebral, but not myocardial blood flow51,73 , but clinical studies have failed to demonstrate convincing benefit97 . Simultaneous ventilation might be more effective in barrel-chested breeds of dogs and large-sized animals when it is difficult to generate an effective increase in intrathoracic pressure by conventional external compression. Simultaneous ventilation and chest compression is not recommended as a first-order therapy because of its potential for inducing barotrauma and pneumothorax.
Internal chest compression
Internal heart compression, compared to external chest compression, is associated with better cardiac output, arterial blood pressure, cerebral and coronary perfusion, and myocardial perfusion, peripheral tissue perfusion, higher mixed-venous PO2, less arterial and mixed-venous metabolic acidosis, lower mixed-venous lactate concentrations, higher mixed-venous and end-tidal PCO2, and higher survival rates with improved neurological recovery14 . During closed chest compression coronary blood flow is particularly difficult to achieve because when the chest is compressed, aortic pressure and right atrial pressure increase equally; there is no pressure gradient across the heart and therefore there is no coronary blood flow33,72 . Myocardial blood flow may, perhaps, occur when the chest is released, during the brief period of time when (if) aortic pressure is higher than right atrial pressure. Open-chest, direct cardiac compression, compared to continued closed-chest CPR, after 15 minutes of closed-chest CPR, was associated with a 100% vs 36% resuscitation rate, and a 79% vs 29% 7-day survival rate in dogs52 . This is not to say, however, that a thoracotomy should be done in every resuscitation. The heart perhaps can be started with external compression without an invasive thoracotomy, performed under much less than ideal circumstances. It is to say, however, that thoracotomy and direct cardiac compression should be considered to be a valid CPR technique which should be applied to the appropriate patients. In one veterinary study, a thoracotomy was performed in 23% of 135 dogs and in 14% of 43 cats49 . A spontaneously beating heart was re-established in six dogs (16% of the 38 subjected to the technique) and one cat (6% of the 18 subjected to the technique).
There are other advantages to a thoracotomy: 1) The adequacy of diastolic filling can be assessed. The heart should fill as rapidly as it is released. If this does not occur, it is objective evidence of the lack of venous return and the need for a fluid bolus or an alpha-receptor agonist. 2) The presence of an accumulation of fluids or blood within the pericardial sac can be observed. The pericardial sac can be opened to prevent pericardial tamponade during or subsequent to resuscitation. 3) The descending aorta can be depressed with the index finger of the opposite hand or clamped, directing essential blood flow to the brain and heart. If this option is chosen, the compression should remain for the duration of the resuscitation. It should be removed only after the spontaneous activity has been determined to be stabile, and then should be removed gradually over 10 to 20 minutes. 4) Ventricular fibrillation can be diagnosed by direct observation and internal defibrillation efforts may be more effective than external. 5) Myocardial flaccidity can be assessed by direct visualization.
A thoracotomy is indicated when there is an open or a closed pneumothorax, when there is chest trauma with broken ribs, or when the size or shape of the thorax precludes effective external chest compression techniques. A thoracotomy should be performed if there is no evidence of forward blood flow with the external compression techniques within 5 minutes. A thoracotomy should be performed if, after 10 minutes, there is no spontaneous heart beat. A thoracotomy is not a procedure to be used as a last resort after everything else has failed to restart the heart. Delayed thoracotomies do not improve return of spontaneous circulation statistics 88,93 .
Time is limited yet the thoracotomy needs to be done accurately so as not to generate additional life-threatening complications. Clip a strip of hair along the line of the intended incision at the fifth intercostal space. Remove hair and lose dirt with one swab of an antiseptic solution. Make the incision midway between the ribs down to, but not through, the pleura. Avoid the caudal edge of the rib - there are big intercostal vessels here that bleed a lot when cut. Pleural penetration should be accomplished with something blunt, like a finger. The incision is then extended dorsally and ventrally with scissors taking care to avoid the internal thoracic artery which runs longitudinally about one centimeter lateral to the sternum.
Small hearts can be compressed between two fingers; larger hearts between the flats of the fingers and the palm of the hand; and still larger hearts between the palm and the opposite chest wall. Care must be exercised not to use the fingertips (which might penetrate the wall of an atrium or ventricle) and not to rotate the heart (which might cause inflow occlusion) or displace the heart cranially or caudally (which might tear the vena cava-right atrial junction). The rate of compression should be coordinated to the rate of ventricular refill, i.e., compress again as soon as the ventricle has refilled.
Fluids
Cardiac arrest is a rapidly vasodilating disease process secondary to tissue anoxia. The increasing blood volume capacity must be filled with exogenous fluids to maintain an effective central circulating blood volume. Notwithstanding pre-existing anemia or hypoproteinemia, a crystalloid fluid such as lactated Ringer's, should be administered rapidly intravenously in aliquots of approximately 40 ml/kg for the dog and 20 ml/kg for the cat. This bolus volume may need to be repeated periodically throughout the resuscitation endeavor in quantities sufficient to maintain an effective circulating volume. Excessive fluid volumes predispose to pulmonary edema and should be avoided. 7.5% hypertonic saline solution (6 ml/kg), 20 to 25% mannitol (10 ml/kg), or an artificial colloid solution (10 ml/kg) (these are dog dosages) could also be used.
Sympathomimetics
All sympathomimetics with alpha agonist activity, with or without beta agonist activity are efficacious in CPR75,82 . Venoconstriction redistributes blood from the venous capacitance vessels into the active arterial circulation. Arterial constriction diminishes the loss of fresh cardiac output into the periphery. Isoproterenol and dobutamine are not recommended because of their peripheral vasodilating properties. Beta agonist activity stimulates pacemaker activity and enhances contractility. Epinephrine is the preferred drug because it generates the greatest coronary and cerebral blood flow and is associated with the best resuscitation rates 17,18,61,64 . Norepinephrine and phenylephrine might be considered in situations in which one needs peripheral vasoconstriction without the beta-receptor mediated fibrillation effect. There are, however, alpha receptors in the heart, and they can mediate fibrillation as well. Vasopressin 62 and angiotensin II 60 are effective vasoconstrictors as well;.
The dose of epinephrine is 0.02 to 0.2 mg/kg3,19,20,27 . High dosages may cause ventricular fibrillation 11 and this is a problem if the facility does not have a defibrillator. Pharmacological defibrillation techniques are not uniformly effective. It is prudent to start with the low dose and titrate to higher doses with successive administrations in an attempt to maximize the good effects of epinephrine without inducing the bad effects.
Vasopressin (antidiuretic hormone) in large dosages (0.5 mg/kg IV) causes a V1-receptor (nonadrenergic) mediated vasoconstriction which may improve many cardiovascular parameters during CPR and may increase the incidence of return of spontaneous circulation 1 . Vasopressin may be used as an alternative to epinephrine. Vasopressin may be more effective in prolonged CPR endeavors when the vasculature becomes non-responsive to epinephrine62,106 . Vasopressin has a long half-life (10-20 minutes) and so does not need to be re-administered during the CPR endeavor.
There are four routes by which small volume emergency drugs can be administered: central venous, peripheral venous, intra-cardiac, and intratracheal. A jugular catheter with its tip in the anterior vena cava, close to the right atrium, is ideal because it is close to the heart and it avoids the problems inherent in the other routes. Peripheral veins are the most commonly available. The problem with peripheral venous routes of small drug administrations is the time delay associated with their delivery to the heart and lower blood levels compared to the central venous route7,50 . Peripheral veins closer to the heart (cephalic) are preferable to veins further away (saphenous) or intra-osseous, but any intravenous access is better than no access. Blind intracardiac injections may be associated with lung, coronary artery, or atrial laceration. Intra myocardial deposition of epinephrine may cause refractory ventricular fibrillation. Multiple myocardial injections cause trauma and may predispose to ventricular ectopic pacemakers or ventricular fibrillation. The intracardiac route is not a first choice route for these reasons, however, neither is it a non-choice. The intratracheal route has been recommended 81,83 but drug uptake is entirely dependent upon local blood flow, which is unpredictable during cardiac arrest resuscitation 47,65,79,111. The drug is still probably better in the trachea than it is still in the syringe. Double the dose that would have be given intravenously and then add enough saline to have a sufficient volume with which to work. Pass a long urinary catheter and deposit the drug at the level of the carina. Sometimes, when attempting a blind intracardiac puncture, the only thing one can find is lung. There is no research data to support this next comment, but it is this author's opinion that an "intra-lung" injection is at least equivalent to an intra-tracheal drug deposition.
Anticholinergics
The use of an anticholinergic is unquestionably indicated for the treatment of severe bradycardia. It is not so clear during CPR, bit excessive vagal tone and the lack of an idioventricular (escape) rhythm can cause and maintain asystole16,95 . Atropine may also potentiate sinus tachycardia or ventricular fibrillation if given in the vicinity of epinephrine. Atropine (0.04 mg/kg) should be administered once, early in the resuscitation.
Sodium bicarbonate
Sodium bicarbonate is administered during CPR to combat the metabolic acidosis generated by anaerobic metabolism in hypoxic tissues. Dogs develop a moderate to severe mixed-venous and cerebral metabolic acidosis within 15 minutes during cardiac arrest with active resuscitation 87 . Resuscitation was more prompt and 24 hour neurologic recovery was better when the metabolic acidosis is controlled 36,58,96,101 The efficacy of sodium bicarbonate is, however, controversial, because, if misused, can contribute to the problem1,6,41,104,105 .
When administered liberally, sodium bicarbonate can cause severe metabolic alkalosis. Increased mortality was reported when the pH exceeded 7.55105 . The administration of sodium bicarbonate is also associated with the generation of carbon dioxide (via the carbonic acid-bicarbonate buffer system) resulting in hypercapnia if the patient is not well ventilated. Carbon dioxide rapidly diffuses into the intracellular compartment and into the CSF. Once inside, it re-equilibrates across the carbonic acid equilibrium, generating an excess of hydrogen ion92 . Intracellular acidosis may be associated with myocardial and CNS depression12,28 . It is imperative to make sure that these patients are well ventilated so that the generated carbon dioxide is eliminated.
There are alternative alkalinizing agents. Tromethamine (THAM) binds directly with hydrogen ion which decreases rather than increases carbon dioxide levels. Carbicarb is an equimolar combination of sodium carbonate and sodium bicarbonate. Sodium bicarbonate, tromethamine and Carbicarb improved both survival and myocardial performance in the postresuscitation period38,96 . Sodium bicarbonate has not been demonstrated to lack efficacy nor have these alternative agents been demonstrated to be superior.
Sodium bicarbonate administration increases plasma sodium concentration and osmolality. The increase is moderate and similar to that associated with the administration of hypertonic saline.
Sodium bicarbonate is recommended at a dose of 0.5 mEq/kg per 5 minutes of cardiac arrest after the first 5-10 minutes if the patient had a normal acid-base balance at the time of the arrest, or right from the start, if it is suspected that the animal had a moderate to severe metabolic acidosis prior to the arrest. Do not use the formula: base deficit x 0.3 x kg because this is calculated to treat the metabolic acidosis of the entire extracellular fluid compartment and would cause severe hypotension if administered as an intravenous bolus. This dose would have to be administered over a period of 20 to 30 minutes, to allow time for it to redistribute into the interstitial fluid compartment.
Calcium
Calcium has been reported to diminish resuscitation rates if the heart is fibrillating or in asystole 44 and is not recommended for routine use during CPR1 . It may, however, be efficacious for some subsets of electromechanical dissociation. Intracellular calcium concentrations increase rapidly during myocardial hypoxia108 . Excessive intracellular calcium concentrations can cause sustained muscular contraction of the heart. The use of calcium during resuscitation may also cause coronary and cerebral artery vasoconstriction, which further diminishes blood flow to the heart50,69 . Calcium is also an early player in the generation of toxic oxygen radicals and its use may contribute to cell injury by this mechanism as well50,89,108 .
Calcium is, however, one of the specific treatments for life-threatening hyperkalemia and is essential if this is the attributed cause of the cardiac arrest. It also is recommended in cases of severe hypocalcemia and in some cases of electromechanical dissociation. It is also recommended if the chest is open and the heart is observed to be flabby and atonic. It might also be recommended at the end of the drug list, when all other pharmacologic endeavors have failed to achieve a spontaneously beating heart. The recommended dose is 0.2 ml of 10% CaCl or 0.6 ml of 10% CaGluconate per kilogram of body weight.
Magnesium
Intravenous magnesium sulfate may be efficacious in hypomagnesemic patients with refractory arrhythmias including ventricular fibrillation1,66 .
Antiarrhythmics
Lidocaine (1 to 3 mg/kg IV) may be useful to reduce the heterogeneity of ventricular refractoriness and may occasionally be useful after CPR if the ventricular arrhythmias are multiform, frequent in occurrence (> 180/min), increasing in frequency, or are associated with impaired cardiac output. Lidocaine is not effective for supraventricular tachyarrhythmias. Lidocaine may increase the defibrillation threshold34,56 .
Procainamide (1 to 3 mg.kg) may be effective in both ventricular and supraventricular arrhythmias.
Amiodarone has emerged as a preferable antiarrhythmic in ventricular fibrillation/tachycardia1 . It is also effective for supraventricular arrhythmias. It is not that lidocaine has been shown to be ineffective, but the nature of its antiquated evidence simply does not push it to the top in an evidence-based review1 . Amiodarone blocks sodium, potassium, and calcium channels, and blocks alpha and beta receptors. The intravenous dosage in humans is 2-4 mg/kg, administered over 10 minutes, and then approximately 0.5 to 1 mg/kg/hr1 . Prolonged use may be associated with a pneumonitis.
Corticosteroids?
There have been very few studies regarding the use of corticosteroids in cardiac arrest. One early report suggested that steroids improved survival in patients with electromechanical dissociation107 . Hydrocortisone was also reported to significantly improve return of spontaneous heart beat in a rat KCl-induced cardiac arrest model94 . The Guidelines 20001 , gives it a class IIb designation which means that corticosteroids should be considered acceptable and useful in the treatment of vasopressor resistant states but with only fair to good evidence to support the recommendation.
Don't give glucose
Glucose should only be administered to treat hypoglycemia. Modest hyperglycemia following resuscitation in both dogs 32 and cats68 , diminished neurological outcome. The mechanism is attributed to enhanced intracellular lactic acidosis.
Electrical activity of the heart
Electrocardiographic monitoring during the resuscitation endeavor is important because it helps define the type of cardiac arrest at that moment and helps to guide some of the therapeutic interventions (Table 1). There are three forms of cardiac arrest and a typical resuscitation will see all three. 1) Ventricular fibrillation is characterized by chaotic electrical and mechanical heart activity. This rhythm needs to be defibrillated; it will not spontaneously resolve. The most reliable means of defibrillation is direct electric current, but in the absence of such equipment, pharmacologic defibrillation should be attempted. 2) Ventricular asystole is characterized by the lack of electrical or mechanical heart activity. This form of cardiac arrest requires an endogenous pacemaker: a beta-agonist drug like epinephrine. 3) Pulseless electrical activity denotes the presence of rhythmic electrical activity, but without sufficient mechanical heart activity to generate a detectable pulse. In humans, the 10 most common causes of pulseless
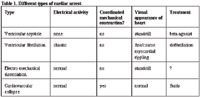
Different types of cardiac arrest
electrical activity are hypovolemia, hypoxia, acidosis, a potassium abnormality, severe hypothermia, cardiac tamponade, tension pneumothorax, coronary thrombosis, pulmonary thrombo-embolism, and drug overdosage1 . Narrow, fast rhythms associated with a definable/treatable cause have a fairly good prognosis. Successful resuscitation when there are wide, slow rhythms associated with an unidentifiable cause require a miracle. Underlying, intrinsic heart disease (hypertrophic/dilative cardiomyopathy, valvular insufficiency or stenosis, fibrosis) downgrade the chances for a successful resuscitation.
All forms of cardiac arrest require the restoration of myocardial oxygenation. Myocardial oxygenation is dependent upon the creation of an adequate mean arterial blood pressure. Given the shape of the pulse pressure contour during CPR (very narrow; variably tall), mean pressure is very close to diastolic pressure. The creation of mean/diastolic arterial blood pressure is dependent upon optimal venous return, effective cardiac/thoracic compression, and vasoconstriction.
There is no question that ventricular fibrillation is a bad thing when it occurs. Ventricular fibrillation is exercising muscle, in which metabolic rate and oxygen demands are high. At the same time vascular resistance is high, and arterial blood pressure, perfusion pressure and oxygen delivery is low. Survival decreases 7-10% per minute and is virtually zero after 12 minutes43,57,67,71,111 .
A critical quantity of energy is required to successfully defibrillate a heart. The only problem is that you will not know in advance what it is. Start with 2-5 J/kg (external). Make sure to have good, broad paddle contact. If this does not succeed, try three rapid sequence defibrillations. If this does not work, try higher settings on subsequent attempts. The fibrillation algorithm in this chapter suggests a sequence of increasing power settings for external defibrillation of 5, 10, and 15 J/kg. Pre-oxygenation, epinephrine, and chest compression may diminish myocardial hypoxia and facilitate successful defibrillation29,71 . If this does not succeed, perhaps internal defibrillation (0.2-0.3 j/kg) might. Excessive energy and repeated defibrillations can, however, cause myocardial damage. It is desirable to start with lower settings and to find the effective setting as soon as possible. In human adults, for monophasic (one direction of current flow) waveform defibrillators, the recommended dose of the first shock (or series of shocks) is 200 J; 300 J for the second shock; and 360 J for the third103 . The newer biphasic waveform (two, opposite-directioned, current pulses) defibrillators are more efficient and require less energy8,78 .
Transthoracic impedance to current flow is an issue with external defibrillation and is the reason why recommended external power settings are 10 times higher than those for internal. Transthoracic impedance can be reduced and the effectiveness of the defibrillation technique enhanced by: 1) clipping the hair; 2) using relatively larger paddles (or electrodes); 3) liberal use of electrolyte coupling material; 4) pressing the paddles more firmly against the chest wall; 5) compressing the thorax between the paddles and defibrillating while the lungs are deflated so that the distance between the electrodes is minimized.
The use of automated external defibrillators (AEDs) by lay bystanders has substantially improved survival rates in humans67,109 . The units are being made available for public access on commercial airlines and in airports and shopping malls, etc., because the preponderance of evidence suggests that public assess defibrillation will become an indispensable component of the goal of early defibrillation in the "chain of survivial"1,22 . AEDs are simple-to-use, computerized devices that analyze the electrical rhythm of the patient and advises the operator when a shock is indicated. The units typically attach to the patient via large, self-adhesive electrode pads (animals would have to be shaved first).
If direct-current defibrillation is not available, pharmacological defibrillation can be attempted, but this is not reliable. Potassium chloride (1 mEq/kg) followed by calcium (0.2 ml/kg 10% CaCl or 0.6 ml/kg 10% CaGluc), with or without the pre-administration of lidocaine (1 mg/kg). A sharp thump to the chest may, rarely, restore a normal rhythm1 .
Follow-up care
Perhaps the most important part of a resuscitation procedure is the quality of the follow-up care: 1) identification and treatment of the underlying disease process; 2) maximizing pulmonary function; 3) optimizing cardiovascular function; 4) minimizing neurological deterioration; and 5) normalizing laboratory abnormalities (electrolytes, anemia, coagulopathies) and other organ function (renal, gastrointestinal).
Reperfusion injury
First there is the lack of perfusion injury. The lack of oxygen and other metabolic substrates disenables the cell's ability to maintain energy stores for essential cellular functions. Such cells lose the ability to pump sodium outward and develop intracellular edema.
It has been observed that in some cases the brain and heart seem to function reasonably well immediately after resuscitation, but then deteriorate in the ensuing hours108 . There are several reasons for this: 1) reperfusion injury associated with the generation of reactive oxygen intermediates91 , 2) leucocyte sequestration and activation associated with the generation of many inflammatory mediators such as tumor necrosis factor, platelet activating factor, and various proteases)23 and 3) disseminated intravascular coagulation15,77 .
Reactive oxygen metabolites (superoxide anion radical, hydrogen peroxide, and hydroxyl anion) have an odd number of electrons (either one too many or one too few). They are very chemically reactive molecules, either donating (oxidation) or extracting (reduction) the spare electron from neighboring molecules and cell membranes. The two primary sources of reactive oxygen metabolites are: 1) in the catabolism of ATP to hypoxanthine, and 2) the activation of neutrophils. Free ferrous iron (which also accumulates in the cells during the ischemic period) catalyzes the conversion of superoxide anion and hydrogen peroxide to the hydroxyl anion radical54,108 . Reactive oxygen metabolites cause lipoperoxidation of cell and organelle membranes, oxidize sulfhydryl groups, activate (or inactivate) enzyme systems (impaired calcium transport, decreased phosphocreatine, activated collagenases degrade basement membranes, activated hyaluronidases degrade interstitial matrix, and myocardial contractility is impaired), and cause DNA and RNA depolymerization. There is no proven way to deal effectively with these oxygen metabolites in clinical patients.
Pulmonary function
First evaluate the animal's ability to ventilate; hypercapnia must be avoided46 . Apnea is due to a centrally mediated neurologic dysfunction; the animal will require positive pressure ventilation until such time as the neurologic disease can be reversed. Mannitol is indicated to osmotically reduce the probable cerebral edema. Furosemide may be indicated if a large volume of crystalloids was administered during the resuscitation, and particularly if there is any evidence of edema. Ineffective ventilatory efforts could be attributed to pneumothorax, tracheal tube/airway fluid accumulation, or severe pulmonary contusion/edema.
Ineffective oxygenation could be due to pulmonary edema from aggressive fluid therapy or to pulmonary contusions from the chest compressions; hypoxemia must be avoided. Oxygen therapy should be provided as a matter of routine and especially if there is any evidence of hypoxemia.
Pneumonia was reported to occur in 28% of human patients within 7 days84 . Gram-positive organisms (mostly Staph. aureus) accounted for 57% of the infections, 33% were due to Pseudomonas, while 17% were polymicrobial.
Cardiovascular function
Cardiovascular function should be intensely monitored and supported following return of spontaneous circulation: 1) myocardial performance; 2) arterial blood pressure; and 3) adequacy of tissue perfusion.
Myocardial cells rapidly develop a severe hypercarbic acidosis during cardiac arrest due to in situ bicarbonate buffering of metabolic acids 48 and significant myocardial systolic and diastolic dysfunction occurs following resumption of a spontaneously beating heart25,38,53 . This myocardial stunning may either spontaneously resolve within 48 hours or may cause progressive myocardial failure. If poor contractility is suspected, a beta-agonist sympathomimetic should be administered. Dobutamine (5 to 15 mcg/kg/min) might be an ideal drug to improve myocardial function and cardiac output while causing mild peripheral vasodilation, as long as arterial blood pressure is maintained above 80 mmHg. Dopamine (5 to 15 mcg/kg/min) may be useful if low arterial blood pressure is an issue. Bradycardia (< 50 to 60 beats/min) becomes a problem when it is associated with a decrease in cardiac output, arterial blood pressure, or tissue perfusion. An anticholinergic should be administered (atropine: 0.02 to 0.04 mg/kg; glycopyrrolate: 0.01 to 0.02 mg/kg). If this is not effective, perhaps a sympathomimetic would be effective (dopamine: 3 to 7 mcg/kg/min; dobutamine: 5 to 10 mcg/kg/min; epinephrine: 0.05 tp 0.1 mcg/kg/min).
Hypotension is associated with inadequate perfusion of the brain and the heart. The mean blood pressure must be maintained at least above 80 mmHg. Hypotension may be due to hypovolemia, reduced cardiac output, or peripheral vasodilation. Hypovolemia should be corroborated by a measurement of central venous pressure. Careful fluid therapy with crystalloids, colloids, or blood products should be considered. Poor contractility may require sympathomimetic therapy (dobutamine or dopamine). Peripheral vasodilation may be associated with residual tissue hypoxia and accumulations of metabolic acids and cytokines. Alpha receptor agonists (norepinephrine or phenylephrine) may be indicated initially to pharmacologically support blood pressure2 . Hypertension may represent the Cushing's response to elevated intracranial pressure and probably should not be reduced by the administration of vasodilators.
The electrocardiogram should be monitored. Arrhythmias are anticipated following a resuscitation. They do not need to be treated if they are not progressing in severity or are likely to convert to ventricular fibrillation, and if they are not interfering with cardiac output and blood pressure. Specific treatment of ventricular ectopic pacemaker activity is indicated when the arrhythmia is severe: 1) when the rate exceeds (or would if the paroxysmal rhythm continued for an entire minute) 180 to 200 beats per minute; 2) when the arrhythmia is multiform in nature; 3) when the incidence or severity is becoming greater or more severe; 4) when the ectopic foci fires during the T wave of the preceding complex; or 5) when there is evidence of inadequate cardiac output. Total elimination of the arrhythmia is not the objective of therapy since, many times, the adverse effects of the antiarrhythmic drug occur prior to conversion to normal sinus rhythm. A simple decrease in the rate or severity of the arrhythmia may be a suitable end-point to the titration of the antiarrhythmic drugs.
The adequacy of tissue perfusion is assessed by the usual clinical and laboratory signs. Optimization of fluid therapy and venous return must be assured. Mean arterial blood pressure should be maintained above 80 mmHg to assure adequate cerebral and coronary perfusion pressure.
Neurologic recovery
Cardiac arrest produces cerebral hypoxia in 10 seconds, depletes glucose and glycogen stores in 2-4 minutes and depletes ATP stores in 4-5 minutes. The energy deficient cell accumulates sodium, calcium, and iron, and dies. Post-resuscitation cerebral failure has been attributed to: 1) global ischemia and ATP depletion, 2) reperfusion injury, and 3) extra cerebral derangements (hypotension, hypoxemia, acidemia, absorbed gut toxins, sepsis, coagulopathies, activated complement and prostaglandin cascades, and other organ failures)25 . Initially, following return of spontaneous circulation, there are multifocal areas of "no-reflow" followed by a phase of global hyperemia lasting up to 30 minutes, and then a more prolonged period of low flow. This is followed either by return of normal circulation or a delayed nonhomogeneous global hypoperfusion and, finally, brain death35,85,108,110 .
Pre-arrest consciousness should return within 15 to 30 minutes following restarting the heart. If consciousness does not return soon, or if the cardiac arrest time exceeded 15 minutes, the existence of cerebral damage should be assumed. Neurological scoring systems have been devised for use in experimental animals and perhaps these should be adopted for clinical settings14,68 . Trends of neurological competence should be monitored closely in the hours and days following the arrest.
The most important aspect of cerebral resuscitation is to commence effective artificial circulation as early as possible and to achieve effective spontaneous circulation as soon as possible. Following resuscitation of spontaneous circulation, the most important aspects of cerebral resuscitation involve the physiologic management and support of the patient. Avoid hypotension, hypercapnia, hypoxia, and hyperthermia. Avoid venous outflow obstruction and head-down positioning. Hyperventilation decreased intracranial pressure for several hours and improved neurologic recovery in one study99 , but may compound the naturally occurring reduced blood flow period following return of spontaneous circulation, diminishing neurologic recovery 4,86,112 and is therefore not recommended.
In an 11-minute, no flow, canine cardiac arrest model, moderate hypertension, mild hemodilution, normocapnia and mild hypothermia (34C) improved neurologic recovery and reduced histopathologic brain damage compared to the normotensive, hypocapnic, and normothermic group86 . Hyperoxia diminished neurological recovery in one study 113 .
Hyperthermia/fever cannot not be allowed to occur. Cerebral metabolic rate is directly proportional to temperature (approximately 7-8% per degree centigrade (2 F) change in temperature). Tissue damage accrues when the oxygen requirement exceeds oxygen delivery. Moderate hypothermia (32-34 C [90-90 F]) may improve neurologic outcome55,59,63 . Mild levels of post-arrest hypothermia should not be actively treated.
Mannitol (0.5 g/kg; administered slowly intravenously) osmotically decreases cerebral edema and scavenges hydroxyl radicals. Dimethylsulfoxide (1 gm/kg; diluted to less than a 10% solution and administered over 2 hours) may have beneficial effects on neurologic recovery in global ischemia; but did not in one study5 . Thiopental, despite early promising reports, has not been found to be beneficial in cerebral resuscitation. Iron chelators (deferoxamine [25-50 mg/kg]) may become an important therapeutic modality in cerebral resuscitation. Pentoxifylline diminished postischemic hypoperfusion of the cerebral cortex in a feline cardiac arrest model98 .
References
1. American Heart Association, Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care: International Consensus on Science,. Circulation 2000;102:I-1 - I-384.
2. Angelos MG, Ward KR, Beckley PD, Norepinephrine-induced hypertension following cardiac arrest: effects on myocardial oxygen use in a swine model. Ann Emerg Med, 1994;24:907-914.
3. Angelos MG, DeBehnke DJ, Epinephrine and high-flow reperfusion after cardiac arrest in a canine model. Ann Emerg Med, 1995;26:208-215.
4. Ausina A, Baguena M, Nadal M, et al, Cerebral hemodynamic changes during sustained hypocapnia in severe head injury can hyperventilation cause cerebral ischemia? Acta Neurochir Supple 1998;71:1-4.
5. Badylak SF, Babbs CF, Kougias C, Blaho K, Effect of allopurinol and dimethylsulfoxide on long-term survival in rats aftr cardiopulmonary arrest and resuscitation, Am J Emerg Med 1986;4:313-318.
6. Barzilay Z, Somekh, E, Sagy M, Boichis H, Pediatric cardiopulmonary resuscitation outcome, J Med 1988;19:229-241.
7. Barsan WG, Levy RG, Weir H, Lidocaine levels during CPR: Differences after peripheral venous, central venous, and intracardiac injections. Ann Emerg Med 1981;10:73-78.
8. Bardy GH, Marchlinski FE, Sharma AD, et al, Multicenter comparison of truncated biphasic shocks and standard damped sine wave monophasic shocks for transthoracic ventricular defibrillation, Circulation 1996;94:2507-2514.
9. Bayes de Luna A, Coumel P, Leclercq JF, Ambulatory sudden cardiac death: mechanisms of production of fatal arrhythmia on the basis of data from 157 cases, Am Heart J 1989;117:151-159.
10. Bedell SE, Delbanco TL, Cook EF, Epstein FH, Survival after cardiopulmonary resuscitation in the hospital. N Eng J Med 1983;309:569-576.
11. Berg RA, Otto CW, Kern KB, Sanders AB, Hilwig RW, Hansen KK, Ewy GA, High-dose epinephrine results in greater early mortality after resuscitation from prolonged cardiac arrest in pigs: a prospective, randomized study. Crit Care Med, 1994;22:282-290.
12. Berenyi KJ, Wolk M, Killip T, Cerebrospinal fluid, acidosis complicating therapy of experimental cardiopulmonary arrest. Circulation 1975;52:319-324.
13. Beyar R, Kishon Y, Kimmel E, Intrathoracic and abdominal pressure variations as an efficient method for cardiopulmonary resuscitation: studies in dogs compared with computer model results, Cardiovasc Res 1985;19:335-342.
14. Bircher N, Safar P, Cerebral preservation during cardiopulmonary resuscitation. Crit Care Med 1985;13:185-189.
15. Bottiger BW, Motsch J, Bohrer H, Boker T, Aulmann M, Nawroth PP, Martin E, Activation of blood coagulation after cardiac arrest is not balanced adequately by activation of endogenous fibrinolysis. Circulation, 1995;92:2572-2578.
16. Brown DC, Lewis AJ, Criley JM, Asystole and its treatment: The possible rose of the parasympathetic nervous system in cardiac arrest. JACEP1979;8:448-452.
17. Brown CG, Taylor RB, Werman HA, et al, Myocardial oxygen delivery/consumption during cardiopulmonary resuscitation: A comparison of epinephrine and phenylephrine. Ann Emerg Med 1988;17:302-308.
18. Brown CG, Katz SE, Werman HA, et al, The effect of epinephrine versus methoxamine on regional myocardial blood flow and defibrillation rates following a prolonged cardiorespiratory arrest in a swine model. Am J Emerg Med 1987;5:361-369.
19. Brown CG, Werman HA, Davis EA, et al, Comparative effect of graded doses of epinephrine on regional brain blood flow during CPR in a swine model. Ann Emerg Med 1986;15:1138-1144.
20. Brown CG, Martin DR, Pepe PE, Stueven H, Cummins RO, Gonzalez E, Jastremski M, A comparison of standard-dose and high-dose epinephrine in cardiac arrest outside the hospital. The Multicenter High-Dose Epinephrine Study Group. New Eng J Med, 1992;327:1051-1055.
21. Callaham M, Barton C, Prediction of outcome of cardiopulmonary resuscitation from end-tidal carbon dioxide concentration, Crit Care Med 1990;18:358-362.
22. Callaham M, Madsen CD, Relationship of timeliness of paramedic advanced life support interventions to outcome in out-of-hospital cardiac arrest treated by first responders with defibrillators, Ann Emerg Med 1996;27:638-648.
23. Caceres MJ, Schleien CL, Kuluz JW, Gelman B, Dietrich WD, Early endothelial damage and leukocyte accumulation in piglet brains following cardiac arrest. Acta Neuropathologica, 1995; 90:582-591.
24. Cerchiari EL, Safar P, Klein E, Diven W, Visceral, hematologic and bacteriologic changes and neurologic outcome after cardiac arrest in dogs. The visceral post-resuscitation syndrome.
Resuscitation, 1993;25:119-136.
25. Cerchiari EL, Safar P, Klein E, Cantadore R, Pinsky M, Cardiovascular function and neurologic outcome after cardiac arrest in dogs. The cardiovascular post-resuscitation syndrome. Resuscitation, 1993;25:9-33.
26. Chandra NC, Gruben KG, Tsitlik JE, et al, Observations of ventilation during resuscitation in a canine model, Circulation 1994;90:3070-3075.
27. Choux C, Gueugniaud PY, Barbieux A, Pham E, Lae C, Dubien PY, Petit P, Standard doses versus repeated high doses of epinephrine in cardiac arrest outside the hospital. Resuscitation, 1995;29:3-9.
28. Cingolani HE, Faulkner SL, Mattiazzi AR, et al, Depression of human myocardial contractility with "respiratory" and "Metabolic" acidosis. Surgery 1975;77:427-432.
29. Cobb LA, Fahrenbruch CE, Walsh TR, et al, Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation, JAMA 1999;28:1182-1188.
30. DeBard ML, Cardiopulmonary resuscitation: analysis of six years' experience and review of the literature. Ann Emerg Med, 1981;10:408-416.
31. DeBehnke D, Resuscitation time limits in experimental pulseless electrical activity cardiac arrest using cardiopulmonary bypass. Resuscitation, 1994;27:221-229.
32. D'Alecy LG, Lundy EF, Barton KJ, Zelenock GB. Dextrose containing intravenous fluid impairs outcome and increases death after eight minutes of cardiac arrest and resuscitation in dogs. Surgery 1986;100:505-511.
33. Ditchey RV, Winkler MD, Rhodes CA, Relative lack of coronary blood flow during closed-chest resuscitation in dogs. Circulation 1982;66:297-302.
34. Echt DS, Black JN, Barbey JT, Evaluation of antiarrhythmic drugs on defibrillation energy requirements in dogs: sodium channel block and action potential prolongation, Circulation 1989;79:1106-1117.
35. Fischer M, Hossmann KA, No-reflow after cardiac arrest. Intens Care Med, 1995;21:132-141.
36. Fiser DH, Wrape V, Outcome of cardiopulmonary resuscitationin children, Pediatr Emerg Care 1987;3:235-238.
37. Gazmuri RJ, vonPlanta M, Weil MH, et. al., Cardiac effects of carbon dioxide-consuming and carbon dioxide-generating buffers during cardiopulmonary resuscitation, J Am Coll Cardiol 1990;15:482-490.
38. Gazmuri RJ, Weil MH, Bisera J, Tang W, Fukui M, McKee D, Myocardial dysfunction after successful resuscitation from cardiac arrest. Crit Care Med, 1996;24:992-1000.
39. Gillis J, Dickson D, Rieder M, et. al., Results of inpatient pediatric resuscitation, Crit Care med 1986;14:469-471.
40. Gudipati CV, Weil MH, Bisera J, Expired carbon dioxide: a noninvasive monitor of cardiopulmonary resuscitation. Circulation 1988;77:324-329.
41. Guerci AD, Chandra N, Johnson E, et al, Failure of sodium bicarbonate to improve resuscitation from ventricular fibrillation in dogs. Circulation 1986;74:75-79.
42. Henik RA, Wingfield WE, Cardiopulmonary arrest and resuscitation in cats, Proc Vet Emerg Crit Care Soc, 1987;66-69.
43. Herlitz J, Bang A, Holmberg M, et al, Rhythm changes during resuscitation from ventricular fibrillation in relation to delay until defibrillation, number of shocks delivered and survival, Resuscitation 1997; 34:17-22.
44. Hughes WG, Ruedy JR, Should calcium be used in cardiac arrest? Am J Med 1986;81:285-296.
45. Innes PA, Summers CA, Boyd IM, et. al., Audit of paediatric cardiopulmonary resuscitation, Arch Dis Child 1993;68:487-491.
46. Idris AH, Wenzel V, Becker LB, Banner MJ, Orban DJ, Does hypoxia or hypercarbia independently affect resuscitation from cardiac arrest? Chest, 1995;108:522-528.
47. Jackson RE, The failure of endotracheal epinephrine in cardiac arrest: go for the dough! Acad Emerg Med, 1994;1:328-329.
48. Johnson BA, Weil MH, Tang W, Noc M, McKee D, McCandless D, Mechanisms of myocardial hypercarbic acidosis during cardiac arrest. J Appl Physiol, 1995;78:1579-1584.
49. Kass PH, Haskins SC, Survival following cardiopulmonary resuscitation in dogs and cats, J Vet Emerg Crit Care, 1992;2:57-65.
50. Katz A, Reuter H, Cellular calcium and cardiac cell death. Am J Cardiol 1979;44:188-190.
Kaye W, Bircher NG, Access for drug administration during cardiopulmonary resuscitation. Crit Care Med 1988;16:179-182.
51. Kern KB, Carter AB, Showen RL, Twenty-four hour survival in a canine model of cardiac arrest comparing three methods of myocardial cardiopulmonary resuscitation, J Am Coll Cardiol 1986;7:859-867.
52. Kern KKB, Sanders AB, Badylak SF, Janas W, Carter AB, Tacker WA, Ewy GA, Long-term survival with open-chest cardiac massage after ineffective closed-chest compression in a canine preparation. Circulation, 1987;75:498-503.
53. Kern KB, Hilwig RW, Rhee KH, Berg RA, Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning, J Am Coll Cardiol 1996;28:232-240.
54. Krause GS, Nayini NR, White BC, Hoenher TJ, Garritano AM, O'Neil BJ, Aust SD, Natural couse of iron delocalization and limpid peroxidation during the first eight hours following a 15-minute cardiac arrest in dogs. Ann Emerg Med 1987;16:1200-1205.
55. Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H, Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med, 1993;21:1348-1358.
56. Kupersmith J, Electrophysiological and antiarrhythmic effects of lidocaine in canine acute myocardial ischemia. Am Heart J 1979;7:360-3669.
57. Ladwig KH, Schoefinius A, Danner R, et al, Effects of early defibrillation by ambulance personnel on short and long-term outcome of cardiac arrest survival: the Munich experiment, Chest 1997;112:1584-1591.
58. Ledingham IM, Worman JN, Acid-base studies in experimental circulatory arrest. Lancet 1962; 2:967-969.
59. Lei B, Tan X, Cai H, Xu Q, Guo Q, Effect of moderate hypothermia on lipid peroxidation in canine brain tissue after cardiac arrest and resuscitation. Stroke, 1994; 25:147-152.
60. Little CM, Brown C, Angiotensin II improves myocardial blood flow in cardiac arrest. Resuscitation, 1993;26:203-210.
61. Levesay JJ, Follette DM, Feyk H, et al, Optimizing myocardial supply/demand balance with adrenergic drugs during cardiopulmonary resuscitation. J Torac Cardiovasc Surg 1978;76:244-251 .
62. Lindner KH, Prengel AW, Brinkmann A, Strohmenger HU, Lindner IM, Lurie KG, Vasopressin administration in refractory cardiac arrest. Ann In Med, 1996;124:1061-1064.
63. Marion DW, Leonov Y, Ginsberg M, et al, Resuscitative hypothermia, Crit Care Med 1996;24:S81-89.
64. Michael JR, Guerci AD, Koehler RC, et al, Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation 1984;69:822-835 .
65. McDonald JL, Serum lidocaine levels during cardiopulmonary resuscitation after intravenous and endotracheal administration. Crit Care Med 1985;13:914-915.
66. Miller B, Craddock L, Hoffenberg S, Heinz S, Lefkowitz D, Callender ML, Battaglia C, Maines C, Masick D, Pilot study of intravenous magnesium sulfate in refractory cardiac arrest: safety data and recommendations for future studies. Resuscitation, 199;30:3-14.
67. Mosesso VN, Davis EA, Aubie TE, et al, Use of automated external defibrillators by police officers for treatment of out-of-hospital cardiac arrest, Ann Emerg Med 1998;32:200-207.
68. Nakakimura K, Fleischer JE, Drummond JC, Scheller MS, Zornow MH, Grafe MR, Shapiro HM, Glucose administration before cardiac arrest worsens neurologic outcome in cats, Anesthesiology 1990;72:1005-1011.
69. Nayler WG, et a,: Reperfusion injury: Laboratory artifact or clinical dilemma? Circulation 74:215-221, 1986.
70. Nichols DG, Kettrick RG, Swedlow DB, et. al., Facors influencing outcome of cardiopulmonary resuscitation in children, Pediatr Emerg Care 1986;2:1-5.
71. Nichol G, Stiell IG, Laupacis, et al, A cumulative meta-analysis of the effectiveness of defibrillator-capable emergency medical services for victims of out-of-hospital cardiac arrest, Ann Emerg Med 1999; 34: 517-525.
72. Neimann JT, Differences in cerebral and myocardial perfusion during closed-chest resuscitation. Ann Emerg Med 1984;13:849-853.
73. Neimann JT, Rosborough JP, Niskanen RA, et al, Mechanical "cough" CPR cardiopulmonary resuscitation during cardiac arrest in dogs, Am J Cardiol 1985;55:199-204.
74. Noc M, Weil MH, Tang W, Mechanical ventilation may not be essential for initial cardiopulmonary resuscitation, Chest 1995;108:821-827.
75. Otto CW, Yakaitis RW, Redding JS, et al, Comparison of dopamine, dobutamine, and epinephrine in CPR. Crit Care Med 1981;9:640-643.
76. Pepe PE, Levine RL, Fromm RE Jr, Curka PA, Clark PS, Zachariah BS, Cardiac arrest presenting with rhythms other than ventricular fibrillation: contribution of resuscitative efforts toward total survivorship. Crit Care Med, 1993;21:1838-1843.
77. Pluta R, Lossinsky AS, Walski M, Wisniewski HM, Mossakowski MJ, Platelet occlusion phenomenon after short- and long-term survival following complete cerebral ischemia in rats produced by cardiac arrest. J fur Hirnforschung, 1994;35:463-471.
78. Poole JE, White RD, Kanz KG, et al, Low-energy impedance-compensating biphasic waveforms terminate ventricular fibrillation at high rates in victims of out-of-hospital cardiac arrest, J Cardiovasc Electrophysiol 1997;8:1373-1385.
79. Quinton DW, O'Byrne G, Aitkenhead AR, Comparison of endotracheal and peripheral intravenous adrenaline in cardiac arrest. Lancet 1987;I:828-829.
80. Ralston SH, Babbs CF, Niebauer MJ, Cardiopulmonary resuscitation with interposed abdominal compression in dogs, Anesth Analg 1982;61:645-651.
81. Ralston SH, Voorhees WD, Babbs CF, Intrapulmonary epinephrine during prolonged cardiopulmonary resuscitation: Improved regional blood flow and resuscitation in dogs. Ann Emerg Med 1984;13:79-86.
82. Redding JS, Pearson SW, Evaluation of drugs for cardiac resuscitation. Anesthesiology 1963;24:203-207.
83. Redding JS, Asuncion JS, Pearson JW, Effective routes of drug administratinduring cardiac arrest. Anesth Analg 1967;46:253-258.
84. Rello J, Valles J, Jubert P, Ferrer A, Domingo C, Mariscal D, Fontanals D, Artigas A, Lower respiratory tract infections following cardiac arrest and cardiopulmonary resuscitation. Clin Inf Dis, 1995;21:310-314.
85. Safar P, Effects of the postresuscitation syndrome on cerebral recovery from cardiac arrest. Crit Care Med 1985,13:932-935.
86. Safar P, Xiao F, Radovsky A, et al, Improved cerebral resuscitation from cardiac arrest in dogs with mild hypothermia plus blood flow promotion. Stroke, 1996;27:105-113.
87. Sanders AB, Otto CW, Kern KB, et al, Acid-base balance in a canine model of cardiac arrest. Ann Emerg Med 1988;17:667-671.
88. Sanders AB, Kern KB, Ewy GA, Time limitations for open-chest cardiopulmonary resusciation from cardiac arrest. Crit Care Med 1985;13:897.
89. Schanne FAX, Kane AB, Young EE, et al, Calcium dependence of toxic cell death: A final common pathway. Science 1979;206:700-702.
90. Schindler MB, Bohn D, Cox PN, et al, Outcome of out-of-hospital cardiac or respiratory arrest in children, N Engl J Med 1996;335:1473-1479.
91. Schleien CL, Eberle B, Shaffner DH, Koehler RC, Traystman RJ, Reduced blood-brain barrier permeability after cardiac arrest by conjugated superoxide dismutase and catalase in piglets.
stroke, 1994;25:1830-1834;
92. Shapiro JI, Kucera WR, Kindig KN, Filley G, Chan L, Brain pH responses to sodium bicarbonate and carbicarb during systemic acidosi., Am J Physiol 1989;256:H1316-1321.
93. Sheikh A, Brogan T, Outcome and cost of open- and closed-chest cardiopulmonary resuscitation in pediatric cardiac cardiac arrests, Pediatrics 1994;93:392-398.
94. Smithline H, Rivers E, Appleton T, Nowak R, Corticosteroid supplementation during cardiac arrest in rats. Resuscitation, 1993;25:257-264.
95. Stueven HA, Torsfeldt DJ, Thompson BM, et al, Atropine in asystole: Human studies. Ann Emerg Med 1984;13:815-817.
96. Sun, S, Weil MH, Tang W, Fukui M, Effects of buffer agents on postresuscitation myocardial dysfunction, Crit Care Med 1996;24:2035-2041.
97. Swenson RD, Weaver WD, Niskanen RA, et al, Hemodynamics in humans during conventional and experimental methods of cardiopulmonary resuscitation. Circulation 1988;78:630-639.
98. Tanahashi N, Fukuuchi Y, Tomita M, Kobari M, Takeda H, Yokoyama M, Pentoxifylline ameliorates postischemic delayed hypoperfusion of the cerebral cortex following cardiac arrest in cats. J Neurological Sci, 1995;132:105-109.
99. Vanicky I, Marsala M, Murar J, Marsala J, Prolonged postischemic hyperventilation reduces acute neuronal damage after 15 min of cardiac arrest in the dog. Neuroscience Letters, 1992;135:167-170.
100. VanHoeyweghen RJ, Bossaeert LL, Mullie A, et al, Quality and efficiency of bystander CPR: Belgian Cerebral Resuscitation, Resuscitation 1993;26:47-52.
101. Vukmir RB, Bircher N, Radovsky A, et. al., Sodium bicarbonate may improve outcome in dogs with brief or prolonged cardiac arrest, Crit Care Med 1995;23:515-522.
Weaver WD, Cobb LA, Copass MK, Ventricular defibrillation: a comparative trial using 175-J and 320-J shocks, N Engl J Med 1982;307:1101-1106.
102. Ward KR, Sullivan RJ, Zelenak RR, et al, A comparison of interposed abdominal compression CPR and standard CPR by monitoring end-tidal PCO2, Ann Emerg Med 1989;18:831-837.
103. Weaver WD, Cobb LA, Copass MK, Ventricular defibrillation: a compariative trial using 175-J and 320-J shocks, N Engl J Med 1982;307:1101-1106.
104. Weil MH, Trevino RP, Rackow EC, Sodium bicarbonate during CPR. Does it help or hinder? Chest 1985,88:487.
105. Weil MH, Ruiz CE, Michaels S, et al, Acid-base determinants of survival after cardiopulmonary resuscitation. Crit Care Med 1985;3:888-892.
106. Wenzel V, Lindner KH, Prengel AW, Vasopressin improves vital organ blood flow after prolonged cardiac arrest with postcountershock pulseless electrical activity in pigs, Crit Care Med 2999;27:486-492.
107. White BC, Petinga TJ, Hoehner PJ, Wilson RF, Incidence, etiology, and outcome of pulseless idioventricular thythm treated with dexamethasone during advance CPR. JACEP 1979;8:188-193.
108. White BC, Aust SD, Arfors KE, et al, Brain injury by ischemic anoxia: Hypothesis extension--a tale of two ions? Ann Emerg Med 1984;13:862-867.
109. White RD, Hankins DG, Bugliosi TF, Seven years' experience with early defibrillation by police and paramedics in an emergency medical services system, Resuscitation 1998;39:145-151.
110. Wolfson SK Jr, Safar P, Reich H, Clark JM, Gur D,Stezoski W, Cook EE, Krupper MA, Dynamic heterogeneity of cerebral hypoperfusion after prolonged cardiac arrest in dogs measured by the stable xenon/CT technique: a preliminary study. Resuscitation, 1992;23:1-20.
111. Yakaitis RW, Every GA, Otto CW, et al, Influence of time and therapy on ventricular defibrillation in dogs. Crit Care Med 1980;8:157-163.
112. Yundt KD, Diringer MN, The use of hyperventilation and its impact on cerebral ischemia in the treatment of traumatic brain injury, Crit Care Clin 1997;13:163-184.
113. Zwemer CF, Whitesall SE, D'Alecy LG, Cardiopulmonary-cerebral resuscitation with 100% oxygen exacerbates neurological dysfunction following nine minutes of normothermic cardiac arrest in dogs. Resuscitation, 1994;27:159-170.