Ancillary therapy of respiratory disease in food animals: What can we give in addition to an antibiotic? (Proceedings)
In this session we will take an evidence-based medicine approach to ancillary therapy of bovine respiratory disease. The literature reviewed here is not presented as being all-inclusive, but rather as a summary of many commonly cited articles on these subjects. The citations are primarily peer reviewed, but some are from freedom of information (FOI) summaries and a few are proceedings papers or abstracts.
In this session we will take an evidence-based medicine approach to ancillary therapy of bovine respiratory disease. The literature reviewed here is not presented as being all-inclusive, but rather as a summary of many commonly cited articles on these subjects. The citations are primarily peer reviewed, but some are from freedom of information (FOI) summaries and a few are proceedings papers or abstracts.
The question of ancillary therapy of BRD arises from these data, generated during the National Animal Health Monitoring System (NAHMS) Feedlot Study conducted in 1999.
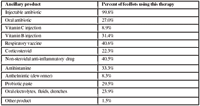
No published data could be found to support the use of Vitamin B or C, vaccines (at the time of therapy), antihistamines, anthelmintics, probiotics, or oral electrolytes in the ancillary therapy of bovine respiratory disease. For the purposes of this presentation, we will examine the published data concerning the use of steroidal and non-steroidal anti-inflammatory drugs as ancillary therapy for respiratory disease.
Glucocorticosteroids? Decades after publication of the study described here, there is still only one published clinical trial addressing the use of steroids for ancillary therapy of BRD as you would encounter it clinically in the United States. One of two treatments was administered to animals identified as displaying clinical signs of BRD. Common drugs for the two treatment groups included IV oxytetracycline (5 mg/lb) and IM pyrelamine (250 mg total dose) on a daily basis for 3 days. Treatment group 1 also received 20 mg dexamethasone every day while treatment group 2 received a 10 ml placebo injection. The same treatments for each group were continued through day 9, as needed, for non-responders. Response was significantly different at P ≤ 0.05 and relapse rate was significantly different at P ≤ 0.01.

These findings aren't that surprising since dexamethasone, at 0.04 mg/kg daily (0.9 ml/100 lbs of a 2 mg/ml solution) for 3 days, is used as a research model to suppress neutrophil function in cattle. This model was utilized in small Holstein calves in conjunction with induced Haemophilus somnus pneumonia to demonstrate that this dexamethasone regimen increased lung lesions. An IBR latency model in rabbits demonstrated that a single high-dose injection of dexamethasone (2.8 mg/kg) could bring about reactivation of latent BHV-1. Other studies have failed to show significant differences in treatment response using prednisone acetate, methyl prednisolone, or methyl-prednisolone-succinate in natural and induced respiratory disease.,
Non-Steroidal Anti-inflammatory Drugs (NSAIDs)
The NSAID currently labeled specifically for BRD in the United States is Flunixin meglumine (Banamine? Injectable Solution, Schering-Plough Animal Health). The label includes indications for the control of pyrexia associated with bovine respiratory disease, acute bovine mastitis, and endotoxemia. The inflammation indication on the label is for the control of inflammation in endotoxemia. Flunixin meglumine is considered an effective analgesic, anti-inflammatory, and antipyretic. The mechanism of action is cyclooxygenase inhibition.
The outlines below summarize published studies and the Freedom of Information (FOI) summaries of flunixin meglumine effects on respiratory disease outcome.
Flunixin BRD study 1: 12 week old dairy calves, induced Pasteurella haemolytica pneumonia 4 treatment groups - no treatment, oxytetracycline (10 mg/kg IM SID for 3 days), flunixin meglumine (2.2 mg/kg IV SID for 3 days), and both oxytetracycline and flunixin meglumine.
Results
• OTC alone reduced the number of calves with fevers and tachypnea and reduced the extent and severity of fibrinous pneumonia as compared to the controls (no mortality)
• Flunixin alone had no antipyretic effect and no reduction in severity of Pasteurella pneumonia compared to the control calves although fewer calves were noted with tachypnea. (1 dead out of 8)
• OTC and flunixin combined had no evident macroscopic consolidation in the lungs, rectal temperature dropped more quickly than in any of the other three groups. (no mortality)
Flunixin BRD study 2: 12-week-old calves, PI3 virus administered into the upper airways 2 treatment groups: Flunixin meglumine (2.2 mg/kg IV SID for 3 days) and controls.
Results: Flunixin reduced the number of calves coughing, the number of calves with fever (> 39.7° C), and the number of calves with tachypnea as compared to untreated controls. There was a marked decrease in pulmonary consolidation in the treated group.
Fluinxin BRD study 3: Calves receiving 3 methylindole (3MI) intratracheally 2 treatment groups: flunixin meglumine (2.2 mg/kg) on the mornings of days 1, 2, and 3 when they started displaying respiratory rates twice that of baseline, and negative controls. Results: Respiratory rates of the treated calves did not significantly differ from that of environmental controls which did not receive the 3MI, while the untreated calves which received 3MI had significantly elevated respiratory rates. Flunixin calves had much less pronounced alveolar epithelial hyperplasia as compared to controls.
Flunixin BRD study 4: Housed beef calves, undifferentiated respiratory disease (a rectal temperature > 39.5° C, respiratory rate > 30/minute, and an increased respiratory effort). Further treatment based on a rectal temperature of 39.5° C on day 4. 2 treatment groups: Tilmicosin phosphate (10 mg/kg SC, once) vs. therapy with tilmicosin phosphate (10 mg/kg SC, once) combined with flunixin meglumine (2.2 mg/kg IV, once). Results: 17/51 tilmicosin calves (27.9 %) required further therapy while 9/58 tilmicosin/flunixin calves (15.5 %) were treated again. This difference was not significant. No mortalities occurred.
Flunixin BRD study 5: Freedom of Information Summary study. 363 calves (heifers, steers, and bulls) 6-12 months old (mean weight 420 lbs.) at 3 locations. Naturally occurring respiratory disease. 2 treatment groups: OTC injectable solution administered for 3 consecutive days at 10 mg/kg (4.5 mg/lb.) IM, as compared to OTC injectable solution (as above) plus flunixin meglumine at 2.2 mg/kg SID for 1-3 days (administered again on days 2 and 3 if temp. not below 104.0° F). Duration of study: 10 days, treatment failure was defined as developing severe recurrent respiratory disease (score of 3 or more on scale of 0=normal, 1=slightly ill, 2=moderately ill, 3=very ill, and 4-moribund) from the end of the 3 days of treatment to the end of the study.
Results
• Mortality – 15/182 dead in OTC alone, 8/181 died in OTC/flunixin group. (One site had 1 mortality in the OTC group, one site had one dead in the OTC/flunixin group, and one site had a concurrent BVD outbreak with 14 dead in the OTC group and 7 dead in the OTC/flunixin group. Not statistically significant
• Character of respiration – No significant difference at pretreatment (day 0) and on day 1. OTC/f group was better on days 2 and 4. On day 9, 99/144 (69%) of the OTC/f calves had normal respiration compared to 70/139 (50%) in the OTC group.
• Illness index scores – More OTC/f animals scored normal on days 1-9. Statistically significant?
• Rectal temperature – Statistically significant difference in temperature decrease on day 1, with OTC/f group having a greater decrease. No significant differences on other study days.
• Depression – Fewer depressed animals in OTC/f group.
• Treatment success/failure – 47/179 (26%) failures in OTC group, 40/181 (22%) in OTC/f group. Not statistically significant.
• Lung pathology – Little or no difference in lung pathology between groups
• Weight gain and daily feed intake – OTC group numerically superior to OTC/f group (16.5 lbs. Vs. 10.6 lbs. respectively) but this was not significantly significant.
Flunixin BRD study 6: Freedom of Information Summary #2. 81 male Holstein calves (304 months, mean 82.3 kg) with naturally occurring BRD (acute clinical signs of pneumonia with elevated rectal temp ≥104.0 ° F and respiratory rate ≥ 40/minute). 2 treatments: OTC injectable solution administered for 3 consecutive days at 10 mg/kg (4.5 mg/lb.) IM, as compared to OTC injectable solution (as above) plus flunixin meglumine at 2.2 mg/kg SID for 1-3 days (administered again on days 2 and 3 if temp. not below 104.0° F).
Results
• Number of flunixin treatments – 58.1% of OTC/f calves required one flunixin meglumine injection, 34.9% required a second, 7% required a third.
• Mortality – No deaths during the study
• Character of respiration – For days 2-7, more animals in OTC/f group had normal respiration than did the OTC group. Statistically significant?
• Illness index scores – More normal animals in OTC/f group on days 2 and 3. Statistically significant.
• Rectal temperature – Statistically significant advantage to OTC/f group on days 1, 2, and 3.
• Depression – Improved demeanor in OTC/f group on days 2-7. Statistically significant?
• Treatment success/failure – 40% treatment failures in OTC/f group, 47% treatment failures in OTC group. 44% of the OTC treatment group occurred on day 3, while 24% of the OTC/f treatment failures occurred on day 3.
• Weight gain – 4.6 kg for OTC/f, 4.0 KG for OTC. Not statistically significant.
What about other NSAIDs for ancillary therapy of BRD? If there is published evidence that phenylbutazone or aspirin change therapeutic outcome in BRD therapy I have not been able to find it. The pharmacokinetics, published anti-inflammatory effects in cattle, and dosing strategies of these compounds in cattle have been previously summarized. Practitioners should be aware that the residue potential of phenylbutazone in cattle is coming under increased scrutiny.
Meloxicam is a member of the oxicam class of NSAID's. The elimination half time is 24 to 26 hours which has resulted in a "long-acting" label claim in the European Union. A recent report evaluating Meloxicam has provided, to our knowledge, the first evidence of a demonstrated economic benefit to using a NSAID in the treatment respiratory disease in feedlot cattle. Animals with clinical symptoms of BRD received 20mg/kg oxytetracycline with either 0.5mg/kg meloxicam or 0.9% isotonic saline. To assess performance animals were weighed at 0, 7, 35, 70 and 105 days and finally before slaughter. Approximately 200 cattle with a mean body weight of 232kg were evaluated. Mean body weight was significantly higher for the meloxicam treated cattle from Day 70 (p<0.05) until slaughter (p<0.01). The mean average daily gain until slaughter was significantly higher with 1.23 kg in the treated group compared with 1.12kg in the control group (p<0.01). The mean carcass weight of the meloxicam treated group was significantly greater than the control group (P<0.05; 282.1kg vs 269.8 kg). It was concluded that a single injection of meloxicam as adjunct therapy in BRD in feedlot cattle resulted in a substantial pharmaco-economic benefit.
In 2009, Meloxicam was approved in Canada for "...an aid in improving appetite and weight gains when administered at the onset of diarrhea, in combination with oral rehydration therapy, in calves over one week of age." Also "for relief of pain following de-budding of horn buds in calves less than 3 months of age".
Carprofen is a propionic acid derivative that demonstrates weak COX binding. This compound demonstrates age dependent pharmacokinetics with significantly longer elimination times is younger cattle. In Europe, the manufacturer claims that a single injection provides relief for up to 3 days in calves less than 12 months of age. A clinical trial was undertaken to investigate the efficacy of a single dose of carprofen (CPF) in the treatment of bovine respiratory disease in cattle. Tilmicosin was used as a basal treatment in all animals. Six hours after dosing, body temperature and respiratory rates in animals treated with CPF-tilmicosin had decreased and were significantly lower than in the animals treated with tilmicosin alone (P < 0.05). Over the period of clinical observation, CPF-tilmicosin treatment produced a clinical resolution of the pneumonia similar to treatment with tilmicosin alone.
A study comparing treatment of naturally occurring respiratory disease treated with ceftiofur alone or in combination with carprofen, flunixin meglumine or ketoprofen has been published. All three treatments significantly reduced pyrexia during the first 24 hours of the study as compared to the antimicrobial alone. There was less lung consolidation in the NSAID treated groups (statistically significant only in the flunixin group), but there was no significant difference in depression, illness scores, dyspnea, or coughing.
UN, WHO address public health concern over avian flu transmission to humans
April 18th 2024Veterinary professionals working with certain animals are advised to take precautionary steps to minimize risk of infection, while researchers in Texas study potential H5N1 vaccines, antivirals, and antibody therapies for humans
Read More